Description
Accession
Q05030
Source
Optimized DNA sequence encoding extracellular domain of rat PDGFR beta ncluding a C-terminal 6His tag was expressed in HEK293 cells.
Molecular weight
Recombinant rat PDGF-R beta is a monomer protein consisting of510 amino acid residue subunits,due to glycosylation migrates as an approximately 100 kDa protein on SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant PDGFR beta is lyophilized from a 0.2 μm filtered PBS solution, pH7.2.
Storage
The lyophilized protein is stable for at least 2 years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at 2° - 8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Biological Process
Molecular function
Molecular function
Molecular function
Methods
-
Oligodendrocyte. Cells were seeded at a concentration of 5,000 cells/cm2 on tissue culture plastic plates and coverslips and cultured in high glucose DMEM supplemented with 1% Penicillin/Streptomycin , 2 mmol/lL -Glutamine , 1X N1 supplement , 1 µg/ml biotin , 5 ng/ml bFGF , 1 ng/ml PDGF , and 30% B104-conditioned media for 1 day. - On the second day, CG4 rat oligodendrocyte progenitor cells were added in a co-culture setting to promote differentiation, using co-culture membrane inserts, and the media were changed every 2 days.
- The cells were allowed to differentiate for 5 days and were then fixed and stored in PBS for immunofluorescence.
- Cells were subsequently assessed for expression of the oligodendrocyte markers O2 and NG2.
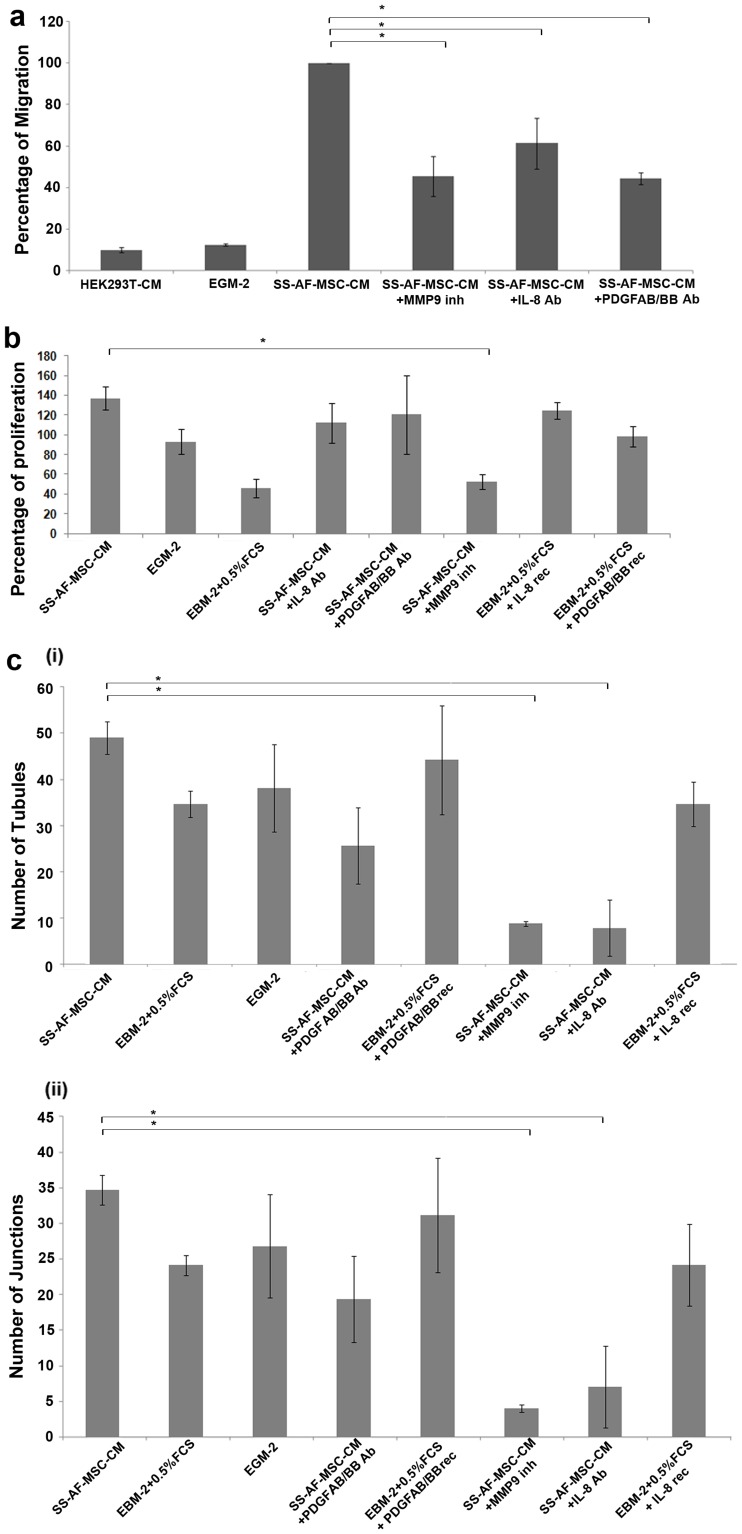
Role of IL-8, PDGF-AB/BB and MMP9 molecules from conditioned media in migration, proliferation and ability of tubule formation of UCB ECFC derived cells.
- Control medium with recombinant (rec) IL-8 or PDGF-AB/BB was also included.
Preparation of SLCs
- After being subcultured at a concentration of 1 × 106 cells/cm2, BM-MSCs were incubated in αMEM containing 1 mM BME without serum for 24 h. The culture medium was then replaced with αMEM containing 10% FBS and 35 ng/ml at-RA .
- After three days, the cells were finally transferred to inducer medium containing αMEM, 10% FBS and trophic factors of 5 μM FSK , 10 ng/ml bFGF , 5 ng/ml PGF , and 200 ng/ml HG .
- The cells were cultured for 10 days [
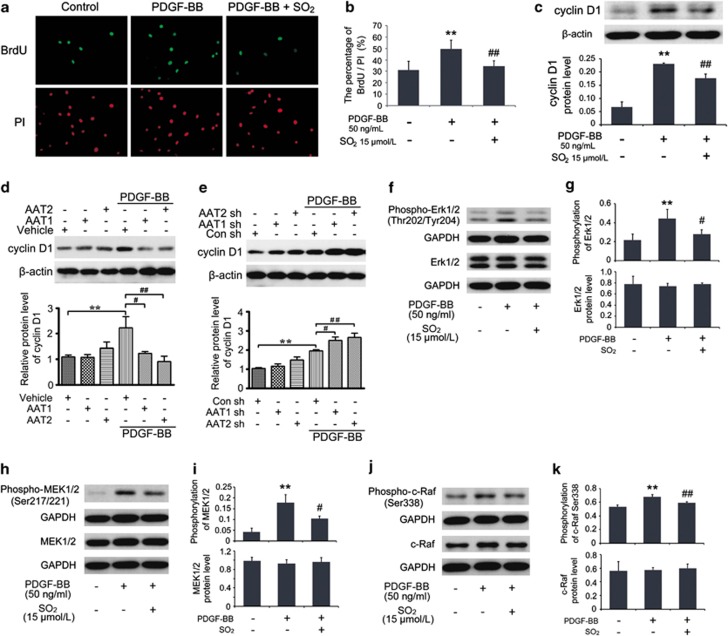
SO2 derivatives suppressed VSMCs proliferation by inhibiting Erk/MAPK pathway.
- Cells in coverslips were starved for 24 h and then pretreated with or without Na2SO3/NaHSO3 at 15 μmol/l for 30 min, as well as with PDGF-BB at 50 ng/ml treatment for 24 h for immunofluorescence assay of BrdU incorporation.