Description
Accession
P24071
Source
Optimized DNA sequence encoding extracellular domain of Rat CD89including a C-terminal 6His tag was expressed in HEK293 cells.
Molecular weight
Recombinant Rat CD89(FCAR) is a monomer protein consisting of 217 amino acid residue subunits, due to glycosylation migrates as an approximately45-50 kDa protein on SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The activity was tested by immobilized recombinant CD89 (10ug/ml) ability to bind human IgA in functional ELISA.
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Rat CD89is supplied as a 0.2 μm filtered PBS solution, pH7.2 .
Storage
Recombinant CD89, as supplied, can be stored in working aliquots at 2° - 8° C for one month, or at -20°C to-70°Cfor twelve months. Avoid repeated freeze/thaw cycles.
Usage
This product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Molecular function
Molecular function
Methods
Derivation of γδ T Cells
- Human peripheral blood was collected (30 ml) from adult healthy donors after obtaining the IRB approval from the Ohio State University Medical Center and obtaining written consents from donors.
- The ethic committee has also approved the procedure and records are saved in the laboratory logbook.
- Freshly collected blood was processed to isolate peripheral blood mononuclear cells (PBMC) following the similar protocol published earlier
Cell Culture
- Rat vascular smooth muscle cells (VSMCs) were isolated by enzymatic digestion of thoracic aortic media from male Sprague-Dawley rats (250–300g, obtained from Tongji Medical College, HUST) by the method of
TcR expression analysis
- Functional assays for the measurement of degranulation (CD107a/b) were performed as described in
Generation of monocyte-derived dendritic cells (Mono-DC)
- To assess the impact of MSCs and SB623 cells on the maturation of dendritic cells, monocyte-derived dendritic cells were generated in the presence of GM-CSF and IL-4.
- On Day 5, human TNF-α (10 ng/ml) was added to each well with or without MSCs or SB623 cells.
- As previous studies confirmed a role of cyclosporin A in hindering dendritic cell maturation [
Cell culture
- Human CAPs were cultured at a density of 6000 cells/cm2 in medium consisting of equal amounts of IMDM (PAA)/DMEM/Ham's F12 medium containing 5% human serum, 1% penicillin/streptomycin, 20 ng/ml basic fibroblast growth factor and 10 ng/ml epithelial growth factor .
- Human cardiac fibroblasts were cultured in Lung/Cardiac Fibroblasts Basal Medium (Cell Applications, Inc. San Diego, USA) plus supplements (CELL Applications).
- CAR and CD55 expression flow cytometry analysis was performed on CAPs and cardiac fibroblasts of the same passage number.
- Murine HL-1 cells were cultured in Claycomb medium (SAFC Biosciences, Kansas, USA) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 100 µM norepinephrine and 2 mM glutamine.
- Chinese hamster ovary (CHO) cells and CHO cells expressing human CAR (a kind gift of J.M.
- Bergelson, Children's Hospital of Philadelphia, Philadelphia) were cultured in Hams F12
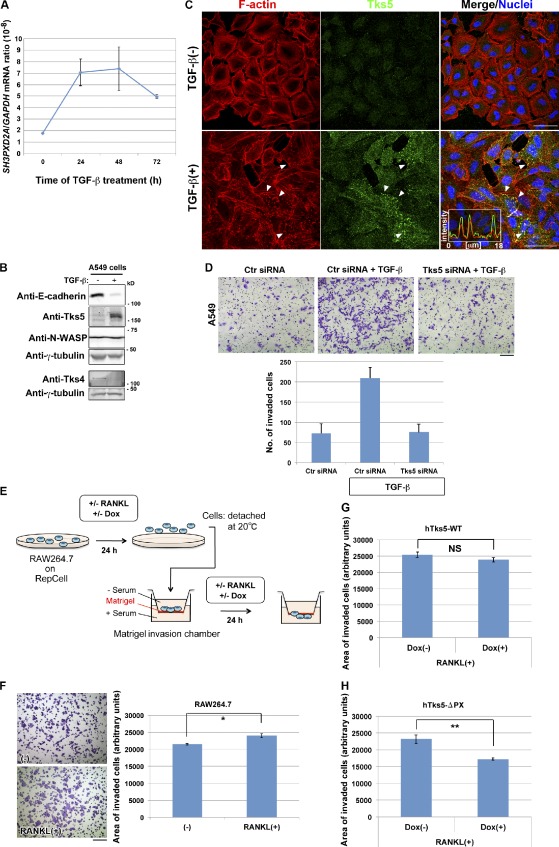
Induction of Tks5 is required for the promotion of invasive activity.
- (A and B) A549 pulmonary carcinoma cells cultured in the presence of 5 ng/ml TGF-β for the indicated times or 72 h were subjected to quantitative RT-PCR analysis of SH3PXD2A mRNA or to immunoblot analysis with the indicated antibodies .
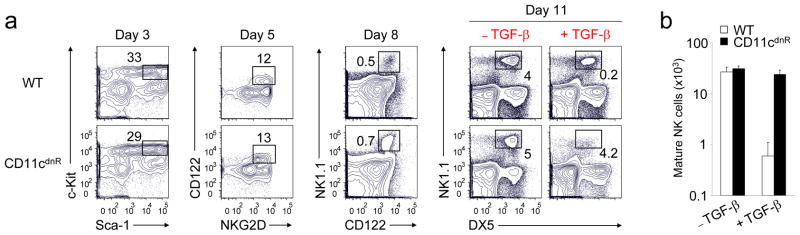
TGF-β is a negative regulator of NK cell generation CD11cdnR and wild-type mice were injected (i.p.)
- On day 8, cultures were supplemented with or without TGF-β and outcomes on NK cell production were determined on day 11.
Oligodendroglial-nanofiber cultures
- 250,000 rat primary oligodendroglial cells isolated from postnatal brains as described above, were cultured on substrate-coated nanofibers in medium'>chemically-defined medium composed of DMEM supplemented with B27 , N2 , penicillin-streptomycin , N-acetyl-cysteine , forskolin and 12.5 ng/ml PDGF-AA .
- Cultures were maintained for 15 days prior to analysis.
-
Definitive endoderm. Differentiation was carried out using the Human Pluripotent Cell-derived Endoderm Differentiation kit (catalog no.SC019& , , ) over 5 days in Activin A-supplemented medium, according to the manufacturer's instructions. - Cells were subsequently assessed for expression of the definitive endoderm markers SOX17, HNF-3β, CXCR4, and GSC and downregulation of the extraembryonic endoderm marker SOX7.
In Vitro Differentiation
- To examine the ability of hBSCs to spontaneously differentiate, breastmilk cells were initially grown as spheroids (see above).
- By day 4–7, some cells had attached.
- The remaining spheroids were transferred into new wells where adherent cells appeared in 1–2 days.
- Both the initial and subsequent attached cells were cultured for another 2–3 weeks, with media changes every 3–5 days.
- For directed differentiation, primary and first- to third-passage breastmilk cell cultures were incubated in differentiation media at 37°C and 5% CO2 for 3–4 weeks.
- For mammary differentiation, cells were incubated in Roswell Park medium'>Memorial medium'>Institute medium (RPMI) 1640 with
l -glutamine supplemented with 20% FBS, 4 μg/ml insulin , 20 ng/ml epidermal growth factor (EGF) , 0.5 μg/ml hydrocortisone , 5% antibiotic-antimycotic, and 2 μL/ml fungizone. - For osteoblastic and adipogenic differentiation, cells were incubated in NH OsteoDiff or NH Adipodiff medium, respectively .
- For chondrocyte differentiation,…