Description
Accession
P97466
Source
Optimized DNA sequence encoding Mouse Noggin mature chain was expressed in Escherichia Coli.
Molecular weight
Native Mouse Noggin, generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calculated molecular mass of approximately 23 kDa. Recombinant Noggin is a disulfide-linked homodimer protein consisting of 2x206 amino acid residue subunits, migrates as an approximately 46kDa protein under non-reducing and as 23kDa under reducing conditions in SDS-PAGE.
Purity
>96%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by induced alkaline phosphatase secretion in ATDC cellsand was determined to be ≤ ng/ml, corresponding to a specific activity of ≥x units/mg.
Protein Sequence
MERCPSLGVT LYALVVVLGL RAAPAGGQHY LHIRPAPSDN LPLVDLIEHP DPIFDPKEKD LNETLLRSLL GGHYDPGFMA TSPPEDRPGG GGGPAGGAED LAELDQLLRQ RPSGAMPSEI KGLEFSEGLA QGKKQRLSKK LRRKLQMWLW SQTFCPVLYA WNDLGSRFWP RYVKVGSCFS KRSCSVPEGM VCKPSKSVHL TVLRWRCQRR GGQRCGWIPI QYPIISECKC SC
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Mouse Noggin was lyophilized from a 0.2 μm filtered solution in PBS, pH.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Biological Process
Biological Process
Molecular function
Methods
Isolation of intestinal crypts
- Tissue was removed from PBS solution and washed multiple times with ice-cold PBS washes until the solution remained clear.
- The specimen was then placed in a Petri dish containing PBS on ice with the mucosal surface facing upward.
- Using a razor blade, excess mucoid material was scrapped from the epithelial surface.
- The specimen was then divided into approximately 0.5 cm2 pieces.
- These pieces were placed into a 2.5 mmol/L EDTA solution in PBS for 30 minutes of incubation with gentle shaking at 4°C.
- After this incubation period, the fragments were allowed to settle and the supernatant was discarded.
- 10 ml of cold PBS was added to the sample, and subsequently vortexed for 10 seconds with 1-second bursts.
- The fragments were allowed to settle, and the supernatant was removed and saved on ice.
- Again 10 ml of PBS were added and the process was repeated eight times.
- Samples were spun down at 100 g for 2 minutes.
- The supernatant…
Monolayer neuronal differentiation
- iPSC cultures were dissociated with Accutase to generate single-cell suspensions, then differentially plated on gelatin-coated plasticware for 1 h in medium'>hESC medium in the presence of ROCK inhibitor (Y27632, Ascent) to remove SNL feeders.
- The nonattached cells were resuspended in MEF-CM (&systems) with 10 ng ml−1 FGF2 and plated on growth-factor reduced Matrigel (B) at a density of 25,000 cells cm−2 and allowed to propagate in self-renewal conditions for 72 h or until 70–90% confluent, whereupon media was changed to KS medium containing 50 ng ml−1 Noggin , 10 μM −1 SHH C24II and 50 ng ml−1 Wnt1 for the second day onwards.
- kk1 blocking antibody (100 ng ml−1& ) was also added for the second day only.
- After 5 days, medium'>medium'>KSR medium'>medium containing these ligands was cross-tapered with medium'>N2B27 medium'>medium (Cells) containing…
Cell culture, transformation assays and luciferase assays
-
All cell lines (NIH3T3, HEK 293, BJ-ET-st
p53 ,p16 , SW480 and HCT-116) were cultured in DMEM supplemented with 10% fetal bovine serum and glutamine/penicillin/streptomycin. - For focus formation assays, NIH3T3 cells were transfected using lipofectamine with 200 ng of pBabe-
Ras V12 plasmid DNA together with 200 ng of pRS shRNA plasmid DNA designed againstNrbp1 (the sequence of shRNA hairpins is provided in the 5 per 6-cm plate and fed every 4 days for 2 weeks and then stained with methylene blue and foci were counted. - Each experiment involved five replicate transfections performed on three separate occasions.
- BJ-ET-st
p53 ,p16 soft agar assays were performed as described previously (U -test. - Luciferase assays were performed in 24-well plates with 7.5 × 103 HCT-116 or HEK 293 cells.
- Cells were cotransfected using lipofectamine with either 200 ng
NRBP1 shRNA vectors or empty vector in combination with either 150 ng M50 Super 8x TOPFlash or M51 Super 8 × FOPFlash and 50 ng…
Crypt isolation and organoid culture
- Organ culture of freshly isolated human small intestinal biopsies was performed in medium'>RPMI medium .
- For organoid culture, crypts were isolated from the small intestine of mice and cultured for a minimum of 7 days as previously described.
- In brief, crypts were isolated by incubating pieces of small intestine in isolation buffer (phosphate buffered saline without calcium and magnesium (PBSO), 2 mM EDTA).
- Crypts were then transferred into matrigel in 48 well plates and 350 μl culture medium (Advanced ME/F12 , containing HEPES (10 mM, PAA), GlutaMax (2 mM), Penicillin (100 U/ml), Streptomycin (100 μg/ml), murine EGF (50 ng/ml), recombinant human-spondin (1 μg/ml& ), N2 Supplement 1x , B27 Supplement 1x , 1 mM N-acetylcystein and recombinant murine Noggin (100 ng/ml)).
- Organoid growth was monitored by light microscopy.
- In some experiments, human biopsies or organoids were treated with recombinant mouse TNF-α (25 ng/ml), recombinant human TNF-α (50 ng/ml),…
Crypt isolation and organoid culture
- Organ culture of freshly isolated human small intestinal biopsies was performed in medium'>RPMI medium .
- For organoid culture, crypts were isolated from the small intestine of mice and cultured for a minimum of 7 days as previously described.
- In brief, crypts were isolated by incubating pieces of small intestine in isolation buffer (phosphate buffered saline without calcium and magnesium (PBSO), 2 mM EDTA).
- Crypts were then transferred into matrigel in 48 well plates and 350 μl culture medium (Advanced ME/F12 , containing HEPES (10 mM, PAA), GlutaMax (2 mM), Penicillin (100 U/ml), Streptomycin (100 μg/ml), murine EGF (50 ng/ml), recombinant human-spondin (1 μg/ml& ), N2 Supplement 1x , B27 Supplement 1x , 1 mM N-acetylcystein and recombinant murine Noggin (100 ng/ml)).
- Organoid growth was monitored by light microscopy.
- In some experiments, human biopsies or organoids were treated with recombinant mouse TNF-α (25 ng/ml), recombinant human TNF-α (50 ng/ml),…
Crypt isolation and culture
- Organoid culture was conducted as previously described. Crypts were released from small intestine tissue fragments by incubation for 30 min at 4°C in 2mM EDTA-PBS solution.
- Isolated crypts were mixed with 50 μl of Matrigel , seeded in 24-well plates and 500 μl of crypt culture medium (MEM/F12 containing 50 ng/ml EGF , 100 ng/ml Noggin and 600 ng/ml-spondin 1 was added.
- Organoids were maintained in a 37 °C humidified atmosphere under 5% CO2.
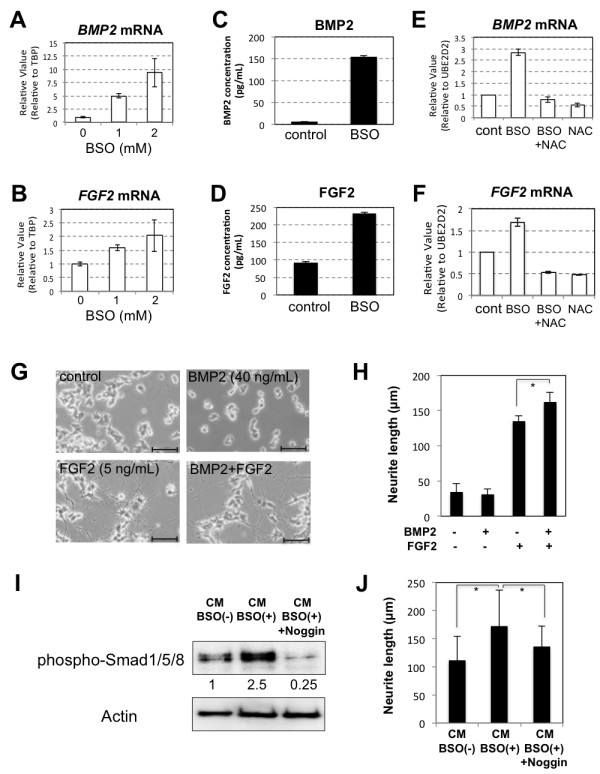
Transcription and secretion of BMP2 and FGF2 were increased in hADMPCs exposed to oxidative stress.
- PC12 cells were cultured in CM-BSO , or CM-BSO added with recombinant murine Noggin (200 ng/mL).
Treatment of AECs with BMP2, 4, 7, noggin and gremlin
- Freshly isolated AECs were seeded onto Oris 96 well migration plates in SCM.
- At day 2, medium was replaced with SFM.
- After 24 hr, supernatant was removed and fresh SFM was added containing appropriate concentrations of recombinant BMP2, 4 and 7 recombinant noggin (ocky , , ) or recombinant gremlin .
Organoid culture
-
SI of mice (
creb ;villinCRE and control littermates; between 3 and 5 weeks old) were isolated, opened longitudinally and cleaned in phosphate-buffered saline (PBS) supplemented with 0.1 mg/ml Nystatin and 0.1 mg/ml Gentamicin (PBS-GN). - The isolated intestines were incubated two times 15 min in PBS-GN on a shaker at 4 °C, followed by incubation in PBS containing 2 mℳ EDTA for 30 min at 4 °C.
- Then the tissues were shaken vigorously in 20 ml cold PBS, of which the supernatant was discarded.
- The tissues were transferred to 10 ml of cold PBS and shaken vigorously again to obtain fraction 2.
- This step was repeated to obtain fraction 3.
- Fractions 2 and 3 were combined and spun down at 200 ×
g for 3 min after which the supernatant, containing single cells, was removed. - The pellet was resuspended in PBS and filtered through a 40-
μ m filter. - The required amount was spun down at 400 ×
g for 6 min. - Five hundred or 1000 viable crypts were seeded in…
Crypt isolation, Enteroid Culture and Analysis
- The pelleted epithelium was re-suspended directly into hESC Matrigel supplemented with 100 ngmL−1 recombinant mouse Noggin , 500 ngmL−1 recombinant human-Spondin , 50ngmL−1 recombinant mouse EGF , 100 ngmL−1 recombinant human Wnt3a (& systems), 10 µM Y-27632 , 10 µM SB202190 , and 500 nM LY2157299 .
- Between 50 and 200 crypt/villi units were plated in 50 uL of matrigel on a 48 well plate.
- After allowing the matrix to polymerize for 30 min at 37°C, each well was overlaid with 500 µL of Advanced DMEM/F12 containing the supplements 1× N-2 supplement , 1× B-27 supplement minus vitamin A , 1× Glutamax , 100 µgmL−1 penicillin/streptomycin, 1 mM Hepes buffer , and 1 mM N-Acetylcysteine.
- Growth factors were added to the media 48 hr after plating and every 72 hr following that.
- The entire volume of media was changed 72 hr following plating and every 72 hr after that.
- Every 1–2 weeks organoids…