Mouse Interleukin-3 Recombinant
Categories: HematopoietinsIL-3 familyRecombinant Mouse Cytokines$70.00 – $4,700.00
Description
Accession
P01586
Source
Optimized DNA sequence encoding mouse Interleukin-3 mature chain was expressed in Escherichia Coli.
Molecular weight
Native mouse Interleukin-3 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 15kDa. Recombinant mouse IL-3 is a monomer consisting of 135 amino acid residue subunits, and migrates as an approximately kDa protein under non-reducing conditions and reducing conditions in SDS-PAGE.
Purity
>98%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation of mouse M-NFS-60 cells was found to be in the <0.05 ng/ml.
Protein Sequence
MVLASSTTSI HTMLLLLLML FHLGLQASIS GRDTHRLTRT LNCSSIVKEI IGKLPEPELK TDDEGPSLRN KSFRRVNLSK FVESQGEVDP EDRYVIKSNL QKLNCCLPTS ANDSALPGVF IRDLDDFRKK LRFYMVHLND LETVLTSRPP QPASGSVSPN RGTVEC
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant mouse Interleukin-3 was lyophilized from a 0.2 μm filtered PBS solution.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Molecular function
Molecular function
Methods
Generation of BMDC and BMMC
- For BMMC induction, 1×106 BM cells were cultured supplemented with 5 ng/ml recombinant murine SCF and IL-3 for more than three weeks (>98% expressed c-kit and FcεRIα).
Mast cell culture from bone marrow
- Mouse bone marrow-derived cultured mast cells (BMCMC) were differentiated from femoral bone marrow by culture in medium supplemented with recombinant mouse IL-3 and stem cell factor(rmSCF, , ) as described −/− bone marrow showed similar granular morphology, levels of active tryptase, and expression of FcεRIα and CD117, indicating that they mature similarly when cultured in the presence of IL-3 and SCF.
Effects of overexpression of Bmi1 on HSCs in vitro.
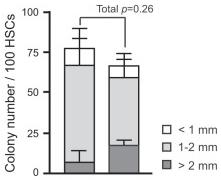
- Single CD34-LSK cells were sorted into 96-well microtiter plates containing the SF-O3 medium supplemented with 10% FBS and multiple cytokines (10 ng/ml SCF, 10 ng/ml TPO, 10 ng/ml IL-3, and 3 u/ml EPO) and allowed to form colonies.
Effects of overexpression of Bmi1 on HSCs in vitro.
- Single CD34-LSK cells were sorted into 96-well microtiter plates containing the SF-O3 medium supplemented with 10% FBS and multiple cytokines (10 ng/ml SCF, 10 ng/ml TPO, 10 ng/ml IL-3, and 3 u/ml EPO) and allowed to form colonies.
Generation and visualization of neutrophil extracellular traps (NETs) from myeloid cell lines and human primary neutrophils.
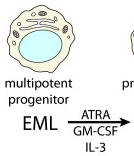
- EPRO cells were generated by differentiating the EML multipotent progenitor cell line with ATRA, GM-CSF and IL-3.
Induction of 2nd iPSCs
- All isolated somatic cells were cultured in the presence of Dox (2 ug/ml) for induction of 2nd iPSCs.
- Fibroblasts and hematopoietic cells were cultured in ES/iPSC medium.
- FLCD45 were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse EPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6, 10 ng/ml mouse Flt3 ligand, 10 ng/ml mouse GM-CSF, 10 ng/ml mouse VEGF and 50 ng/ml mouse SCF .
- HSCs, HPCs and MPs were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6 and 10 ng/ml mouse Flt3 ligand .
- Macrophages were cultured in the presence of 5 ng/ml M-CSF .
Induction of 2nd iPSCs
- All isolated somatic cells were cultured in the presence of Dox (2 ug/ml) for induction of 2nd iPSCs.
- Fibroblasts and hematopoietic cells were cultured in ES/iPSC medium.
- FLCD45 were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse EPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6, 10 ng/ml mouse Flt3 ligand, 10 ng/ml mouse GM-CSF, 10 ng/ml mouse VEGF and 50 ng/ml mouse SCF .
- HSCs, HPCs and MPs were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6 and 10 ng/ml mouse Flt3 ligand .
- Macrophages were cultured in the presence of 5 ng/ml M-CSF .
Biological activities of HoxB4 in Ba/F3 cells.
- 2×103 Ba/F3-HoxB4 cells were cultured with 5 ng/ml IL-3 (survival cytokine) in 96-well plates for 3 days and cell proliferation activity was assessed via MTT assay.
Lentiviral transduction of bone marrow cells
- Freshly isolated bone marrow (BM) cells were plated in Iscove's Modified Dulbecco's Medium (IMDM) (31980, , ) containing 5% fetal bovine serum (FBS), 1% penicillin/streptomycin, 200 mM glutamine, 1% non-essential amino acids, 1% sodium pyruvate, 50 μM 2-mercaptoethanol, stem cell factor (SCF, 10 ng/ml, , ), Flt3-L (10 ng/ml), IL-11 (10 ng/ml), thrombopoietin (TPO, 10 ng/ml), IL-6 (10 ng/ml), and IL-3 (10 ng/ml).
- Bone marrow cells were spin-infected with 8 μg/ml polyprene and lentivirus (MOI of 0.1–0.5), at 2,500 rpm for 90 min.
- BM cells were then incubated for 3 hours at 37°C, counted and injected into NOD.SCID mice.
Generation and isolation of MLL-AF9 leukemia cells
- MLL-AF9 mouse leukemias were generated in Dr. David Scadden's laboratory as described below.
- Actin-DsRED mice (JAX) were backcrossed for 10 generations onto C57BL/6J mice (JAX).
- These mice were sacrificed four days after injection with 150 mg/kg 5FU.
- BM cells were isolated from femurs and tibias, and red blood cells were lysed using ACK lysing buffer .
- Cells were incubated in RPMI supplemented with 20% FBS, 1% Pen-Strep, 6 ng/ml of IL-3 , 10 ng/ml of TPO , 10 ng/ml of IL-6 , at 37°C 5% CO2 overnight.
- MLL-AF9 was introduced by spin-infection (1 000 g for 90 minutes) using a retroviral vector (MSCV-MLL-AF9-neo) 6 live cells per mouse were injected into lethally irradiated (9Gy) C57BL/6 recipient mice 12 hours after viral infection.
- The mice were sacrificed once they became moribund.
- BM cells were isolated as described above, and subjected to ACK lysis.
- 200 000 cells were injected into sublethally irradiated (4.5 Gy)…