Mouse Interleukin-2 Recombinant
Categories: HematopoietinsIL-2 familyRecombinant Mouse Cytokines$70.00 – $2,500.00
Description
Accession
P04351
Source
Optimized DNA sequence encodingMouse Interleukin-2 mature chain was expressed in Escherichia Coli.
Molecular weight
Native Mouse Interleukin-2, generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately17 kDa. Recombinant IL-2 is a monomeric protein consisting of150 amino acid residue subunits, and migrates as an approximately17 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED50 as determined by the dose dependent stimulation of murine CTLL-2 cells is <.1 ng/ml, corresponding to a specific activity of > x units/mg.
Protein Sequence
MYSMQLASCV TLTLVLLVNS APTSSSTSSS TAEAQQQQQQ QQQQQQHLEQ LLMDLQELLS RMENYRNLKL PRMLTFKFYL PKQATELKDL QCLEDELGPL RHVLDLTQSK SFQLEDAENF ISNIRVTVVK LKGSDNTFEC QFDDESATVV DFLRRWIAFC QSIISTSPQ
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant mouse IL-2 was lyophilized from.2 μm filtered PBS solution, pH7.0.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Biological Process
Molecular function
Molecular function
Methods
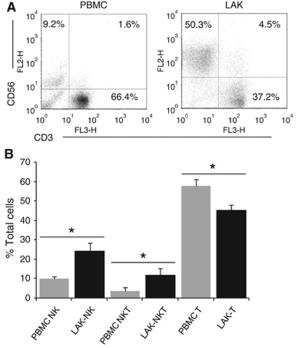
CTL and NK cell assays
- Splenic mononuclear cells were prepared from individual mice one week after immunization.
- The cells were cultured at 37°C for 2 h and non-adherent cells were used as NK effector cells.
- To prepare CTL effector cells, splenic mononuclear cells (5×106) were co-cultured with 5×105 of mitomycin C (50 µg/mL, 40 min) inactivated B16 cells in 5 mL RPMI 1640 containing 10% FBS and 20 units/mL of recombinant IL-2 .
- Five days later, the cells were harvested, purified by Ficoll gradient centrifugation and used as CTL effector cells.
- The NK and CTL-mediated cytotoxicities were determined by lactate dehydrogenase (LDH) release assay.
- Briefly, Yac-1 cells at 2×104/well were cultured in quadruplicate with the prepared NK at the ratios of 12.5, 25, and 50 effector to target cells, respectively in 5% FBS phenol-free RPMI 1640 in 96-well plates at 37°C for 4 h.…
-
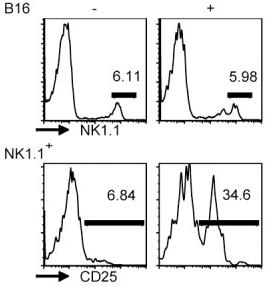
Foxp3 DEREG mice were injected with 0.75 μg of DT in PBS i.p. - on days −2 and −1, and on days 2, 5, and 8 after RSV infection to induce and maintain Foxp3+ T-cell depletion.
- IL-2 Cx were obtained by mixing 1 μg rmIL-2 and 5 μg anti-IL-2 ( eBioscience, San Diego, CA), and incubating at 37°C for 30 min.
- Age- and sex-matched BALB/c or C57BL/6 mice received daily i.p.
- injections of IL-2 Cx or PBS for three consecutive days.
- In some experiments, mice were additionally injected i.p.
- with 50 μl of rabbit anti-mouse asialo GM1 on days −2, 0, and 3 , or with 200 μl of anti-CD25 antibody (PC61; 1 mg ml−1) on days −1 and 3.
Cytokine Assays
- ELISAs were also performed to assess levels of interleukin (IL)-2, IL-4, IL-10, and interferon (IFN)-γ in the supernatant of the MLCs on day 4.
- The capture monoclonal antibody (mAb) (JES5-2A5), detection mAb (JES5-16E3), and recombinant standard for IL-10 were from .
- The capture and detection mAbs for IL-2 (JES6-1A12 and JES6-5H4, respectively), IL-4 (BVD-1D11 and BVD-24G2), and IFN-γ (R4-6A2 and XMG1.2) were from .
- Recombinant standards for IL-2, IL-4, and IFN-γ were from .
Passive transfer of experimental autoimmune encephalomyelitis
- Nine days post induction mice were killed and cells were obtained from the spleen and lymph nodes.
- Cells were suspended in Roswell Park medium'>Memorial medium'>Institute medium supplemented with 10% fetal calf serum, 1 mM L-glutamine, antibiotics, MOG35-55 (20 μg/mL) and mouse recombinant IL-2 (50 units/mL, , , ) and incubated for three days in a CO2 humidified incubator.
- Cells were harvested and live cells were separated with Ficoll-Paque (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and injected into naïve mice.
- In control groups of cells extracts, cells were frozen after separation, thawed three times and then injected into naïve mice.
- On the day of inoculation and 48 hours later, 100 ng of pertussis toxin in 0.1 mL saline was also administered by intraperitoneal injection.
- Mice were followed for clinical symptoms of EAE and evaluated according to a 0 to 6 point score (0 represents no clinical signs and 6…
2.7. Mixed Leukocyte Culture and Cytokine Assays
-
An ELISA was also performed to assess levels of interleukin (IL)-2, IL-4, IL-10, and interferon (IFN)-
γ in the supernatant of the MLC on day 4. - The capture mAb (JES5-2A5), detection mAb (JES5-16E3), and recombinant standard for IL-10 were from .
- The capture and detection mAbs for IL-2 (JES6-1A12 and JES6-5H4, resp.
- ), IL-4 (BVD-1D11 and BVD-24G2, resp.
- ), and IFN-
γ (R4-6A2 and XMG1.2, resp.) - were from .
- Recombinant standards for IL-2, IL-4, and IFN-
γ were from .
Viral replication in CD4+ PBL cultures
- To determine the efficiency of HIV-1 replication in peripheral blood CD4+ lymphocytes, we isolated CD4+ cells from buffy coat peripheral blood mononuclear cells (normal blood donors, Red Cross, Ghent, Belgium) by negative selection using paramagnetic beads (MACS , , ).
- After isolation, the cells were cultured in medium'>RPMI medium supplemented with 2 mM L-glutamin, 10% heat-inactivated fetal calf serum, phytohemagglutinin (1 μg/mL , , ), 20 ng/mL IL-2 , 100 U/mL penicillin, and 100 g/mL streptomycin.
- Thereafter, 1 ng of p24 antigen was added to 2,5*105 PBLs, and the culture was spinoculated at 2,300 rpm for 90 minutes at 32°C.
- After centrifugation, the supernatant was removed and the cells were further cultured in RPMI supplemented with 20 ng/mL IL-2.
- HIV replication was monitored at 1, 3, 5, 7, 9 and 11 days post-infection by measuring the amount of p24 antigen present in the supernatants, using a HIV p24 enzyme-linked immunosorbent…
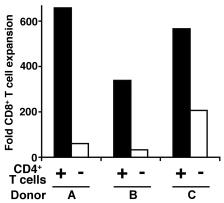
CD4+ T cell cultures
- CD4+ T cells were purified using DynaBeads FlowComp mouse CD4 kit and were activated on αCD3/αCD28 coated plates under the following culture conditions: TH1 conditions: αIL4 10μg/ml (11B11) 0.1μg/ml IFNγ , 10u/ml IL12, 40u/ml; TH2 conditions: αIL12 10μg/ml (Tosh, ), αIFNγ 10μg/ml (XMG1.2, ), 10ng/ml IL4 , 40u/ml; TH17 conditiions: IL-6 25ng/ml , TGFβ 2ng/ml , IL1β 10ng/ml , αIL4 10 μg/ml (11B11, ), αIFNγ 10μg/ml (XMG1.2, ), and αIL12 10μg/ml (Tosh, ).
- IL2 (40u/ml) was added for the TH17 cultures in
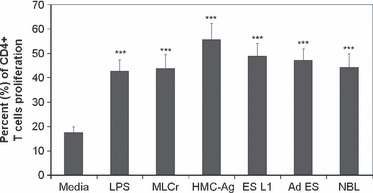
Proliferation of CD4+ T cells cultured with stimulated or non-stimulated BMDCs.
- CD4+ T cells were isolated from mesenteric lymph nodes of C57BL/6 mice, labeled with CFSE and co-cultured for 7 days with BMDCs (non-stimulated, stimulated with LPS or Trichinella spiralis antigens) in the presence of recombinant mouse IL-2 and anti-CD3 antibody.
Treg conversion assay
-
Previously harvested BMDCs (nonstimulated and stimulated with LPS or
T . spiralis antigens) were seeded in 96-well round-bottomed cell culture plates at 2.5 × 103 cells/well.- CD4+ CD25− T cells were added at 5 × 104 cells/well to obtain a final CD4+ CD25− T cell/BMDCs ratio of 20:1.
- OVA (323–339) was added in each well to a final concentration of 0.2 μg/mL.
- Co-cultivated cells were divided into three groups treated with: 40 μg/mL anti-TGF-β (111) antibody as negative control , 2 ng/mL TGF-β as positive control or 40 μg/mL of control mouse IgG .
- On days 1 and 3 of cultivation, rhIL-2 was added in each well to a concentration of 5 ng/mL.
- On day 4, cells were harvested and surface stained for CD4 and CD25, as well as intra-cellular stained for Foxp3 (eBioscience; according to manufacturer’s protocol), as described above.
- Flow cytometry…
Cell analysis and transfection
- For dendritic cell (DC) production, murine bone marrow cells were harvested from femurs of C57BL/6 (B6) mice and plated out in a single-cell suspension in 100ml medium'>RPMI medium (medium'>RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 1mM sodium pyruvate, 2mM L-glutamine, 25mM HEPES buffer, and 50µM 2-ME) in 6 well tissue culture plates (6ml/well).
- GM- CSF was added at 20ng/ml and the cells incubated at 37°C for 7 d. NK cells were isolated from spleens taken from B6 mice using biotinylated anti-CD49b (DX5) antibody ( 553856) together with the CELLection Biotin Binder Kit ( 115–33D).
- NK cells were resuspended at 2 x106 cells/ml in medium'>DMEM medium (medium'>DMEM with 10% FCS, 100U/ml penicillin and streptomycin, 1mM sodium pyruvate, 2mM glutamine, 50µM 2-ME) containing 1000U rIL-2 /ml (212–12) in a 96-well…