Mouse Granulocyte Colony Stimulating Factor Recombinant
Categories: HematopoietinsIL-6 gp130 familyRecombinant Mouse Cytokines$70.00 – $4,700.00
Description
Accession
P09920
Source
Optimized DNA sequence encoding mouse Granulocyte Colony Stimulating Factor mature chain was expressed in Escherichia Coli.
Molecular weight
Native mouse G-CSF is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 19kDa. Recombinant GCSF is a monomer protein consisting of 179 amino acid residue subunits, and migrates as an approximately 19kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent proliferation of murine M-NFS-60 cells is <.05 ng/ml, corresponding to a specific activity of > x units/mg.
Protein Sequence
MAQLSAQRRM KLMALQLLLW QSALWSGREA VPLVTVSALP PSLPLPRSFL LKSLEQVRKI QASGSVLLEQ LCATYKLCHP EELVLLGHSL GIPKASLSGC SSQALQQTQC LSQLHSGLCL YQGLLQALSG ISPALAPTLD LLQLDVANFA TTIWQQMENL GVAPTVQPTQ SAMPAFTSAF QRRAGGVLAI SYLQGFLETA RLALHHLA
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
IRecombinant mouse G-CSF was lyophilized from a 0.2 μm filtered PBS solution.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Molecular function
Molecular function
Methods
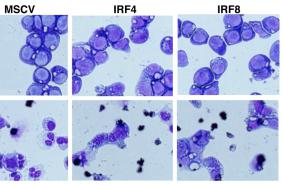
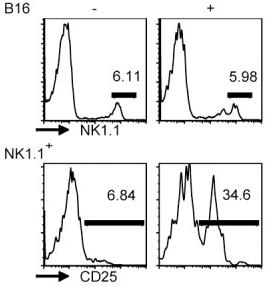
Inhibition of neutrophil differentiation by IRF4.
- Wright-Giemsa staining of 32Dcl.3 cells transduced with empty MSCV-puro, MSCV-IRF4FLAG-puro or MSCV-IRF8FLAG-puro and cultured in the presence of IL-3 (upper panels) or G-CSF (for 7 days, lower panels).
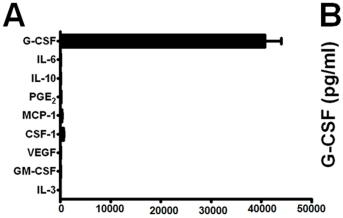
G-CSF production by various mouse tumor models.
- Sera G-CSF levels determined from nontumor-bearing mice (n = 10), mice treated with recombinant G-CSF protein (10 µg daily for 5 consecutive days) (n = 8) or mice bearing 4T1 cells (n = 9) or AT-3 tumor cells pooled from two separate experiments (n = 9; overall tumor volume ≤2cm3).
Bone marrow transduction
- Retroviral transduction of bone marrow was done as previously described (6/ml) were prestimulated for 48 hours in IDMEM containing 10% Premium FBS , 2% Penicillin/Streptomycin, and 100 ng/ml each of recombinant murine TPO, G-CSF, and SCF .
- For primary transduction, cells were resuspended in appropriate retroviral supernatant containing growth factors and seeded onto fibronectin coated wells ((
Generation of MDSCs and DCs from BM
- MDSCs were generated from BM of naïve wt or EGFP-LysM-Tg BALB/c mice.
- Femurs and tibias were collected under aseptic condition, and BM was flushed out with sterile PBS.
- After red blood cell lysis, BM cells were counted (the number of cells was usually 3–4×107 per mouse), and seeded in Petri dishes at a density of 5×105 cells per ml of Dulbecco’s Modified Eagle Medium (DMEM;) containing 10% fetal bovine serum (FBS) .
- In preliminary dose-finding experiments the BM cells were cultured for 3 to 7 days in the presence of varying doses of recombinant murine granulocyte macrophage colony stimulating factor (rmGM-CSF, , ) and recombinant murine interleukin-6 (rmIL-6), or with a combination of rmGM-CSF, rmIL-6, and recombinant murine granulocyte colony stimulating factor (rmG-CSF).
- On the basis of phenotypic and functional characteristics, the optimal protocol for BM-MDSC…
Phenotype and morphology of myeloid-derived suppressor cell (MDSC)-like cells generated in vitro from murine bone marrow (BM) in comparison with synovial fluid (SF) cells.
- BM cells were cultured in the presence of GM-CSF, IL-6, and G-CSF (10 ng/ml each).
Cell culture and retroviral transduction
-
Kasumi-1, 293T and U937 cells were cultured as previously described. G-CSF (granulocyte colony-stimulating factor)-mobilized peripheral blood CD34+ cells were cultured in Iscove's medium'>modified medium'>Dulbecco's medium supplemented with 20% fetal calf serum, 20 ng/ml Flt-3 l, 20 ng/ml GM-CSF, 20 ng/ml stem cell factor (SCF), 20 ng/ml thrombopoietin, 20 ng/ml interleukin (IL)-6, 10 ng/ml IL-3 (all cytokines were obtained, , ), 100 U/ml penicillin/streptomycin and 2 m
M L-glutamine. - Retroviral transduction and long-term cultivation were performed as previously described. IMR-90 cells (ATCC-CCL-186) were cultured as suggested by the supplier.