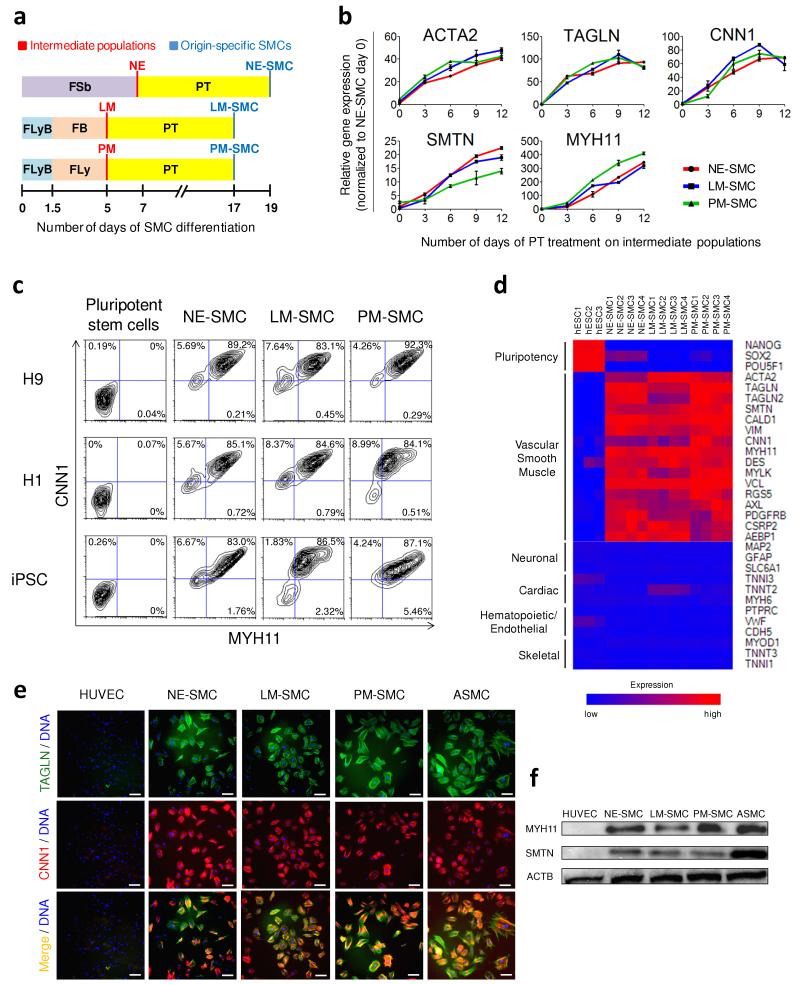
Mouse Glial-Derived Neurotrophic Factor Recombinant
Categories: Neurotrophic factorsRecombinant Mouse Cytokines$70.00 – $4,700.00
Description
Accession
P48540
Source
Optimized DNA sequence encodingMouse Glial Derived Neurotrophic Factor mature chain was expressed in Escherichia Coli.
Molecular weight
Native Mouse GDNF isgenerated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately15 kDa. Recombinant mouse GDNF is a disulfide-linked homodimeric protein consisting of two amino acid residue subunits,and migrates as an approximately30 kDa protein under non-reducing conditions and askDa under reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by theproliferation of rat C6 cells is <.1 ng/ml, corresponding to a specific activity of > x units/mg.
Protein Sequence
MKLWDVVAVC LVLLHTASAF PLPAGKRLLE APAEDHSLGH RRVPFALTSD SNMPEDYPDQ FDDVMDFIQA TIKRLKRSPD KQAAALPRRE RNRQAAAASP ENSRGKGRRG QRGKNRGCVL TAIHLNVTDL GLGYETKEEL IFRYCSGSCE SAETMYDKIL KNLSRSRRLT SDKVGQACCR PVAFDDDLSF LDDNLVYHIL RKHSAKRCGC I
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant mouse GDNF was lyophilized from a 0.2 μm filtered PBS solution.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Molecular function
Methods
Neural precursor culture and viral transduction
- To differentiate NPs to neurons, NPs were plated on polyornithine and laminin coated plates in NP media and grown until they reached 70% confluence.
- FGF was removed and NP media supplemented with 20 ng/ml BDNF , 20 ng/ml GDNF and 0.5 mM dibutyryl cAMP (N6,2′-O-Dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt).
- Medium was exchanged every 2–3 days.
Monolayer neuronal differentiation
- iPSC cultures were dissociated with Accutase to generate single-cell suspensions, then differentially plated on gelatin-coated plasticware for 1 h in medium'>hESC medium in the presence of ROCK inhibitor (Y27632, Ascent) to remove SNL feeders.
- The nonattached cells were resuspended in MEF-CM (&systems) with 10 ng ml−1 FGF2 and plated on growth-factor reduced Matrigel (B) at a density of 25,000 cells cm−2 and allowed to propagate in self-renewal conditions for 72 h or until 70–90% confluent, whereupon media was changed to KS medium containing 50 ng ml−1 Noggin , 10 μM −1 SHH C24II and 50 ng ml−1 Wnt1 for the second day onwards.
- kk1 blocking antibody (100 ng ml−1& ) was also added for the second day only.
- After 5 days, medium'>medium'>KSR medium'>medium containing these ligands was cross-tapered with medium'>N2B27 medium'>medium (Cells) containing…
The SCG SC development assay is free of GDNF.
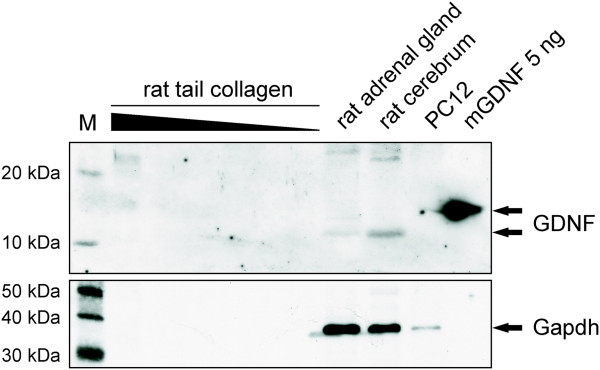
- Western Blot for GDNF with different dilutions of rat-tail collagen (20μl, 10μl, 5μl, 2.5μl, 1.25μl and 0.6125μl collagen) were analyzed using anti-GDNF antibody specific for human and rat GDNF.
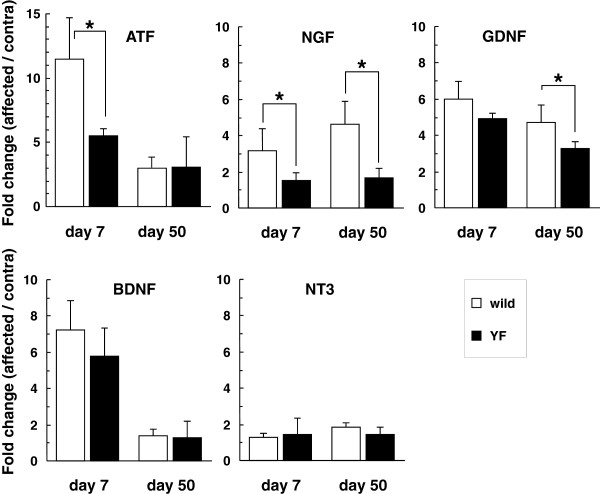
Alterations of gene expression of neurotrophins in the DRG after HSV-1 infection.
- Gene expression levels of ATF3 and neurotrophins (NGF, GDNF, BDNF, and NT3) were normalized to the GAPDH level, and are presented as the fold change in the ratio of the affected side to the contralateral one.
Differentiation of smNPCs
- For generation of more ventral CNS neurons, including mDANs, medium'>smNPC expansion medium was changed 2 days after splitting to N2B27 medium with 100 ng/mL FGF8 , 1 µM PMA, and 200 µM AA.
- After 8 days in this medium, maturation medium–N2B27 with 10 ng/mL BDNF , 10 ng/mL GDNF , 1 ng/mL TGF-b3 , 200 µM AA, and 500 µM dbcAMP–was used for the maturation of neurons.
- 0.5 µM PMA was added to this medium for 2 more days.
- One day after changing to maturation medium, the cultures were split at a 1∶3 ratio as small clumps, or single cells after Accutase treatment, or earlier when cultures became over-confluent.
- Cultures were analyzed after 2 weeks in maturation conditions unless otherwise indicated.
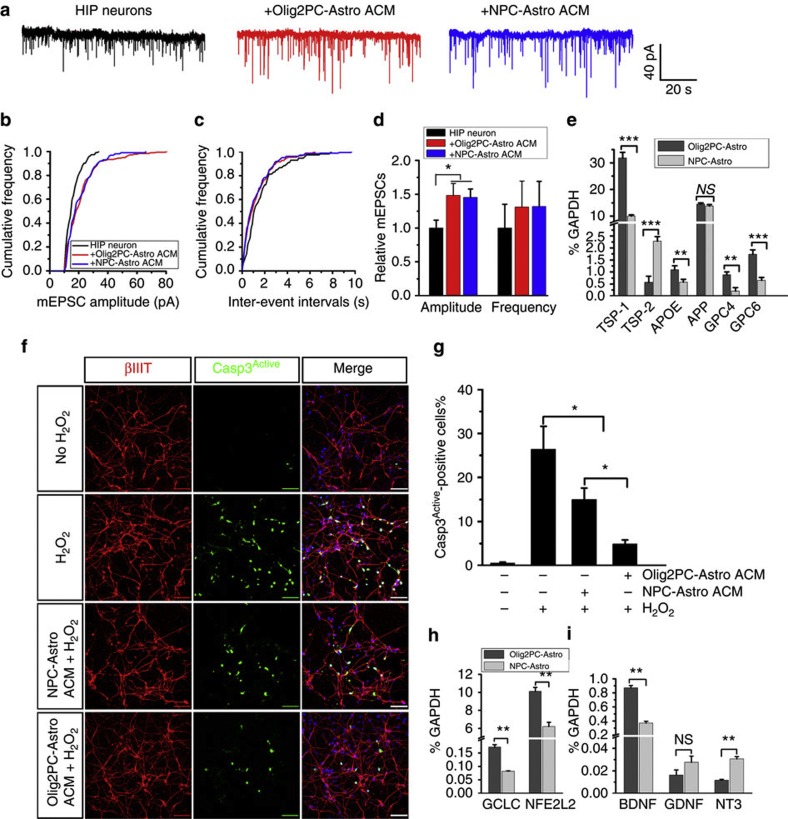
Effects of ACM from Olig2PC-Astros and NPC-Astros on neurons.
- (h and i) RT-PCR analysis of the expression of the antioxidant defense-related genes, GCLC and NFE2L2 (n=3), and the neurotrophic growth factor genes, BDNF, GDNF and NT-3 (n=3).
Neural differentiation of hiPSCs
- Schematic diagram in −1).
- NPCs in the form of rosettes developed for another 1 week (week 2).
- Next, rosettes were manually isolated from surrounding cells and expanded as neurospheres in a suspension culture for 1 week (week 3) in NPC medium, composed of DMEM/F12, 1 × N2, 1 × B27-RA and 20 ng ml−1 FGF2 .
- For neuronal differentiation, neurosphere at week 3 were plated again on growth factor reduced Matrigel-coated plates in medium'>neural medium'>induction medium for 1 week.
- At week 4, only the clusters from which neurons migrated out were picked (−1) pre-coated plates in medium'>neuronal medium'>differentiation medium consisting of DMEM/F12, 1 × N2, 1 × B27-RA, 20 ng ml−1 BDNF , 20 ng ml−1 GDNF , 1 mM dibutyryl-cyclic AMP, 200 nM ascorbic acid .
- Within another week of culture in the neuronal differentiation medium, the majority of the cells showed
Neuronal differentiation of human embryonic stem cells by dual SMAD inhibition.
- 2) Neuronal induction (days 10–20), by incubating cells with brain-derived neurotrophic factor , glia-derived neurotrophic factor , ascorbic acid, dibutyryl-cyclic-AMP (dbcAMP) and the NOTCH1-inhibitor DAPT; 3) Neuronal differentiation (days 20–37), by dissociating cells and re-plating them on poly-lysine/laminin-coated glass coverslips in the presence of BDNF, GDNF and neurotrophin 3 (NT3).
Human embryonic and neural stem cell culture and differentiation
- For neural differentiation, TrypLE Select dissociated single cells were plated onto CELLstart coated Lab-Tek Permanox chamber slides in XF-NSM.
- 24 h after attachment, the media was changed to differentiation media (DM) consisting of X-Vivo 15, 10 ng/mL BDNF , 10 ng/mL GDNF , 1× N2, 1× B27 , 2 ng/mL Heparin (; St. Louis, ), 63 μg/mL NAC , 0.1 ng/mL bFGF, and 10 μg/mL Ciprofloxacin .
- The media was changed every 3 days with half being removed and replaced with fresh DM.
- Differentiation was carried out for a total of 2–4 weeks before cells were permeabilized and immunostained.