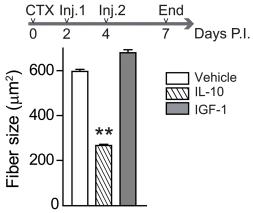
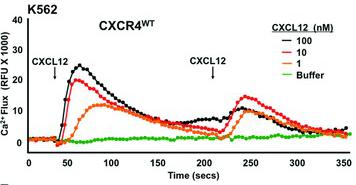
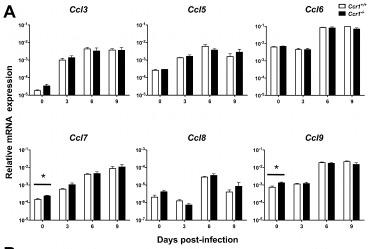
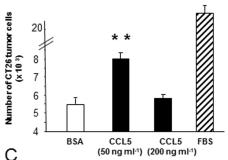
Mouse FLT3 Ligand Recombinant
Category: Recombinant Mouse Cytokines$70.00 – $3,500.00
Description
Accession
P49772
Source
Optimized DNA sequence encoding mouse FLT-3 ligand mature chain was expressed in Escherichia Coli.
Molecular weight
Native mouse FLT-3 Ligand, generated by the proteolytic removal of the signal peptide and propeptide, this molecule has a calculated molecular mass of approximately 19kDa. Recombinant FLT-3 Ligand is a monomer protein consisting of 163 amino acid residue subunits, and migrates as an approximately 19kDa protein under reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent proliferation of human AML5cells was found to be in the range of.0 ng/ml.
Protein Sequence
MTVLAPAWSP NSSLLLLLLL LSPCLRGTPD CYFSHSPISS NFKVKFRELT DHLLKDYPVT VAVNLQDEKH CKALWSLFLA QRWIEQLKTV AGSKMQTLLE DVNTEIHFVT SCTFQPLPEC LRFVQTNISH LLKDTCTQLL ALKPCIGKAC QNFSRCLEVQ CQPDSSTLLP PRSPIALEAT ELPEPRPRQ L LLLLLLLLPL TLVLLAAAWG LRWQRARRRG ELHPGVPLPS HP
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant FLT-3 Ligand was lyophilized from a 0.2 μm filtered solution withmM Tris,100mMNaCl, pH.5.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Molecular function
Methods
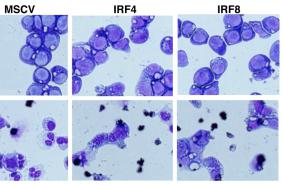
In vitro culture of Eos and MDSCs
- For obtaining Eos, naïve bone marrow cells were cultured in 10% medium'>FBS/PMI medium supplemented with stem cell factor and FLT3-ligand for 4 days, and then with medium containing recombinant mouse IL-5 .
- On day 12, the cells were collected for May-Grünwald-Giemsa staining and flow cytometry analysis6 cells per ml in 10% FBS/RPMI medium, supplemented with 40 ng ml−1 GM-CSF.
- On day 4, the cells were collected for FACS analysis of CD11b+ CD11c− Ly-6C+ Ly-6Glow MDSCs
Induction of 2nd iPSCs
- All isolated somatic cells were cultured in the presence of Dox (2 ug/ml) for induction of 2nd iPSCs.
- Fibroblasts and hematopoietic cells were cultured in ES/iPSC medium.
- FLCD45 were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse EPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6, 10 ng/ml mouse Flt3 ligand, 10 ng/ml mouse GM-CSF, 10 ng/ml mouse VEGF and 50 ng/ml mouse SCF .
- HSCs, HPCs and MPs were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6 and 10 ng/ml mouse Flt3 ligand .
- Macrophages were cultured in the presence of 5 ng/ml M-CSF .
Induction of 2nd iPSCs
- All isolated somatic cells were cultured in the presence of Dox (2 ug/ml) for induction of 2nd iPSCs.
- Fibroblasts and hematopoietic cells were cultured in ES/iPSC medium.
- FLCD45 were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse EPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6, 10 ng/ml mouse Flt3 ligand, 10 ng/ml mouse GM-CSF, 10 ng/ml mouse VEGF and 50 ng/ml mouse SCF .
- HSCs, HPCs and MPs were cultured in the presence of 10 ng/ml human TPO, 10 ng/ml mouse IL-3, 10 ng/ml mouse IL-6 and 10 ng/ml mouse Flt3 ligand .
- Macrophages were cultured in the presence of 5 ng/ml M-CSF .
Lentiviral transduction of bone marrow cells
- Freshly isolated bone marrow (BM) cells were plated in Iscove's Modified Dulbecco's Medium (IMDM) (31980, , ) containing 5% fetal bovine serum (FBS), 1% penicillin/streptomycin, 200 mM glutamine, 1% non-essential amino acids, 1% sodium pyruvate, 50 μM 2-mercaptoethanol, stem cell factor (SCF, 10 ng/ml, , ), Flt3-L (10 ng/ml), IL-11 (10 ng/ml), thrombopoietin (TPO, 10 ng/ml), IL-6 (10 ng/ml), and IL-3 (10 ng/ml).
- Bone marrow cells were spin-infected with 8 μg/ml polyprene and lentivirus (MOI of 0.1–0.5), at 2,500 rpm for 90 min.
- BM cells were then incubated for 3 hours at 37°C, counted and injected into NOD.SCID mice.
Eosinophil counting and culture
- Peripheral blood eosinophils were counted by’s staining [6/ml in IMDM containing 10% FBS , 100 IU/ml penicillin and 10 μg/ml streptomycin , 2 mM glutamine , and 50 μM 2-Mercaptoethanol supplemented with 100 ng/ml stem cell factor (SCF) and 100 ng/ml FLT3 ligand from days 0 to 4.
- On day 4, the medium was replaced with fresh medium containing 10 ng/ml recombinant mouse IL-5 (&systems).
- From this point forward, half of the medium was replaced every other day with fresh medium containing IL-5, and the cell density was maintained around 1×106/ml.
- On day 14, the cells were harvested for
in vitro experiments after flow cytometric identification by CC3-FITC and Siglec-F-PE (B ) staining as well as morphological examination by cytospun slide staining with a modified Giemsa preparation (iff ). - As expected, more than 90% of harvested cells are eosinophils (data not shown).
Culture-derived Generation of Eosinophils from Bone Marrow Precursors
- Ex vivo generation by culture of mouse bone marrow-derived eosinophils was performed essentially as previously described by Dyer et al −/− and mev mice by flushing the bone marrow cavity with RPM 1640 medium .
- After red cell lysis, the bone marrow cells were cultured at 106/ml in medium containing RPMI 1640 with 20% fetal bovine serum (FBS, Cambrex, East Rutherford, ), 100 IU/ml penicillin, 10 µg/ml streptomycin, nonessential amino acids, 1 mM sodium pyruvate (all from , , ), and 50 µM 2-ME supplemented with 100 ng/ml stem cell factor (SCF) and 100 ng/ml FLT3 ligand (FLT3-L) from days 0 to 4.
- On day 4, the cells were moved to new flasks and the medium containing SCF and FLT3-L was replaced with medium containing 10 ng/ml recombinant mouse IL-5 .
- Every other day, from this point forward until cells were used, one-half of the…
AGM region explant culture
- E11.5 AGM regions were dissected and cultured for 5 days on floating 0.8 µm membranes at the liquid-gas interface with IMDM+ media consisting of 20% FCS, , IMDM and growth factors (100 ng/ml IL-3, 100 ng/ml SCF, and 100 ng/ml Flt3 ligand; all from) as previously described (
Lentiviral vectors, LSK-cell transduction, and differentiation and expansion of transduced macrophages
- Transduction, expansion, and differentiation of LSK cells into gene-corrected macrophages was done by adjusting the cytokine ‘cocktail’ mixture to optimize the culture conditions for each of four sequential stages, which included: 1) LSK transduction –murine SCF 50ng/ml, mIL-3 10 ng/ml, hFlt3-L 50 ng/ml and GM-CSF 10 ng/ml), culture time – two 12 hour periods; 2) progenitor expansion - mSCF 50 ng/ml, hFlt3-L 50 ng/ml and GM-CSF 10 ng/ml, culture time - 4 days; 3) macrophage lineage commitment - mSCF 1 ng/ml, hFlt3-L 1 ng/ml, GM-CSF 10 ng/ml, M-CSF 5 ng/ml, culture time - 3 days; and 4) macrophage differentiation - GM-CSF 10 ng/ml and M-CSF 5 ng/ml, culture - 4 days.
- StemSpan containing 2% FBS, 1% penicillin/streptomycin, 10 mM dNTP, and low density lipoprotein was used as the culture medium for the LSK transduction and DMEM with 10% FBS, 50 U/ml penicillin and…
Normal early thymocyte development in CBAP-deficient mice.
- DN thymocytes were isolated from 4-week-old littermates and co-cultured with OP9-DL4 cells in the presence of 1 ng/ml FLT3-ligand and 1 ng/ml IL-7.