Mouse basic Fibroblast Growth Factor Recombinant
Categories: FGF familyFGF familyRecombinant Mouse Cytokines$70.00 – $1,300.00
Description
Accession
P15655
Source
Optimized DNA sequence encoding Mouse basic Fibroblast Growth Factor mature chain was expressed in Escherichia Coli.
Molecular weight
Native Mouse FGF basic is generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calculated mass of 16 kDa. Recombinant mouse bFGF is a monomer protein consisting of 145 amino acid residue subunits, and migrates as an approximately 16 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>98%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of thymidine uptake by BaF3 cells expressing FGF receptors is ≤.2 ng/ml, corresponding to a specific activity of ≥1 x units/mg.
Protein Sequence
MAASGITSLP ALPEDGGAAF PPGHFKDPKR LYCKNGGFFL RIHPDGRVDG VREKSDPHVK LQLQAEERGV VSIKGVCANR YLAMKEDGRL LASKCVTEEC FFFERLESNN YNTYRSRKYS SWYVALKRTG QYKLGSKTGP GQKAILFLPM SAKS
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant mouse basic FGF was lyophilized from.2 μm filtered PBS solution, pH7.0.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Biological Process
Biological Process
Molecular function
Molecular function
Molecular function
Methods
Cell proliferation assay
- MSK543 neurosphere cells grown in NeuroCult NS-A media (Stemcell Technologies, Vancouver, BC, Canada) supplemented with 20 ng/ml EGF (Peprotech, Rocky Hill, NJ), 10 ng/ml bFGF (Peprotech), and 0.0002% Heparin were transfected with 100 nM miRIDIAN miRNA hsa-miR-124, hsa-miR-132, negative control #1, or negative control #2 oligonucleotides (Dharmacon, Lafayette, CO) using the HiPerFect Transfection Reagent (Qiagen, Valencia, CA) according to manufacturer's instructions.
- Cell-cycle analyses were conducted 48 h post transfection using the fluorescein isothiocyanate BrdU Flow Kit following manufacturer's recommendations .
Cell proliferation assay
- MSK543 neurosphere cells grown in NeuroCult NS-A media (Stemcell Technologies, Vancouver, BC, Canada) supplemented with 20 ng/ml EGF (Peprotech, Rocky Hill, NJ), 10 ng/ml bFGF (Peprotech), and 0.0002% Heparin were transfected with 100 nM miRIDIAN miRNA hsa-miR-124, hsa-miR-132, negative control #1, or negative control #2 oligonucleotides (Dharmacon, Lafayette, CO) using the HiPerFect Transfection Reagent (Qiagen, Valencia, CA) according to manufacturer's instructions.
- Cell-cycle analyses were conducted 48 h post transfection using the fluorescein isothiocyanate BrdU Flow Kit following manufacturer's recommendations .
Cell proliferation assay
- MSK543 neurosphere cells grown in NeuroCult NS-A media (Stemcell Technologies, Vancouver, BC, Canada) supplemented with 20 ng/ml EGF (Peprotech, Rocky Hill, NJ), 10 ng/ml bFGF (Peprotech), and 0.0002% Heparin were transfected with 100 nM miRIDIAN miRNA hsa-miR-124, hsa-miR-132, negative control #1, or negative control #2 oligonucleotides (Dharmacon, Lafayette, CO) using the HiPerFect Transfection Reagent (Qiagen, Valencia, CA) according to manufacturer's instructions.
- Cell-cycle analyses were conducted 48 h post transfection using the fluorescein isothiocyanate BrdU Flow Kit following manufacturer's recommendations .
Transfection and reporter gene assays
- 24 h prior to transfection, cells were seeded to 24-well plates.
- For transfections, 15 ng of pRL-TK and either 150 ng promoter construct alone or 100 ng promoter construct and 100 ng expression plasmid were transfected using Lipofectamine 2000 according to the manufacturer's protocol.
- After 6 h, the medium was exchanged into medium'>serum-free medium.
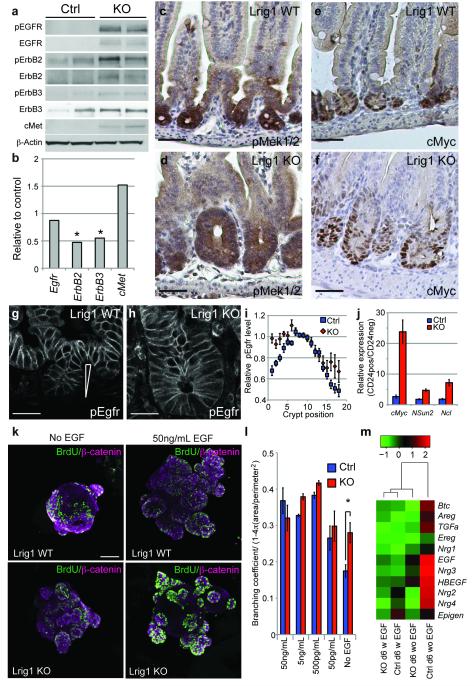
- Treatment with hEGF (10 ng/ml),
trans -retinoic acid (1 µM), phorbol myristate acetate (PMA, 10 ng/ml), MEK1/2 inhibitor U0126 (10 µM , , ), bFGF (10 ng/ml, ), FCS (10%), or troglitazone (1–10 µM) in medium'>serum-free medium was started 24 h after transfection and continued for 24 h. In experiments without stimulation, cells were harvested 48 h after transfection. - Cells were lysed in passive lysis buffer .
- Determination of firefly and renilla luciferase activity was performed with ainfinite M200 plate reader, using 20 µl of lysate and 85 µl of beetle or renilla…
Cell culture
- The hESCs line BG01V was obtained from the ATCC and propagated on mitomycin-C-inactivated mouse embryonic fibroblasts (MEFs;) in hESC medium: Dulbecco's Modified Eagle Medium/F12 (DMEM/F121:1) with 20% knockout serum replacement, 0.1 mM nonessential amino acids, 2 mM L-glutamine, 50 units/ml penicillin G, 50 μg/ml streptomycin sulfate (all from), 0.1 mM beta-mercaptoethanol , and 4 ng/ml bFGF .
- The hESCs were passaged enzymatically every 7 days.
- Briefly, cells were washed with DMEM/F12 (1:1) and then incubated with collagenase IV (1 mg/ml in DMEM/F12) for 40 min at 37 °C and 5% CO2.
- Any semiadhered hESC colonies were pushed off the plate with a plastic pipette, and colonies were sedimented via centrifugation.
- Cell colonies were broken up into smaller cell aggregates by trituration of the harvested colonies in hESC medium.
- Triturated cells were then plated on new mitomycin-C-inactivated MEFs and incubated at 37 °C with…
-
Amniotic fluid was collected between 10 and 12 weeks of gestation ( n = 12) under ultrasound guidance. - Cells were first selected based on plastic adherence until confluency and subsequently selected for c-KIT expression as previously described by us. Cells were expanded (104 cells/cm2) at 37 °C in 5% CO2 on Matrigel in emedia Nutristem XF/FF which sustain the pluripotency state of ES and iPS but do not support the growth of MSC, supplemented or not with VPA (1 mmol/l, , ).
- MSC were from Tulane Center for Gene Therapy (Tulane University, New Orleans, LA).
- celltype'>ES cell lines H1 and H9 (WiCell Research Institute) were cultured in knockout medium'>Dulbecco's medium'>modified medium'>Eagle's medium (KO-DMEM) supplemented with 20% knockout serum replacement, 100 µmol/l nonessential amino acids, 2 mmol/l
L -glutamine, 100 U/ml penicillin/streptomycin, sodium pyruvate, 0.1 mmol/l β-mercaptoethanol , and 8…
-
Ectoderm. AFSC were seeded at a concentration of 3,000 cells/ cm2 on tissue culture plastic plates and coverslips and cultured in DMEM/F12 (1:1) supplemented with 1% Penicillin/Streptomycin, 2 mmol/lL -Glutamine, 0.6% glucose , 3 mmol/l sodium bicarbonate , 5 mM HEPES buffer , 25 mg/ml insulin , 100 mg/ml transferrin , 20 nmol/l progesterone , 60 mmol/l putrescine , 30 nmol/l selenium chloride , 20 ng/ml EGF , 10 ng/ml bFGF , and 10 ng/ml LIF . - Media were changed every 3 days and the cells were allowed to differentiate for 21 days, before being assessed for expression of NESTIN and VIMENTIN.
Genomic Integration and Cell Culture
- RUES2 hESCs were kindly provided by Dr. Ali H. Brivanlou of The Rockefeller University 5 undifferentiated RUES2 hESCs were dispersed to single cells using Versene (Gibco) and electroporated with 5 µg of pAAVS1ZFN and 15 µg of pZDonor mTmG-2a-Puro (Lonza, Kit 1 (Cat#: VAPH-5012), program A-23).
- After electroporation, hESCs were plated onto Matrigel coated plates in mouse embryonic fibroblast (MEF) conditioned media containing 5 ng/mL hbFGF and 10 uM Y-27632 .
- The cultures were maintained for 7 days and then selected with 75 µg/mL G418 in MEF conditioned media for 3 days.
- A polyclonal tdTomato+, G418-resistant RUES2 hESC population was expanded and analyzed for purity by flow cytometry.
- Karyotype analysis was performed by Cell Line Genetics .
- Thereafter, RUES2 mTmG-2a-Puro hESCs showing proper targeting to the AAVS1 locus and a normal karyotype, were maintained under feeder-free conditions, using MEF-conditioned media supplemented with 5 ng/mL hbFGF
Primary Cell Culture
- Culture media is 1×B27 and N2, 1 mM sodium pyruvate, 1 mM L-glutamine , 1 mM N-Acetyl-cysteine in DMEM with 10 ng/ml bFGF , 20 ng/ml EGF , and 2 µg/ml Heparin .
- For experiments, neurospheres were dissociated with NeuroCult ( Cell ) and plated one day prior to analysis on glass bottom dishes coated with 10 ug/ml poly-D-lysine (MP) and 20 ug/ml laminin .
- The protocol to generate cortical NSPCs from mouse embryos is well established and over 95% of the cells are nestin-positive NSPCs
Cell culture
- Human fetal lung fibroblasts (TIG1) provided by the JCRB Cell Bank were cultured in medium'>Dulbecco's medium'>modified medium'>Eagle's medium (DMEM) containing 10% FBS, and were infected with Oct4, Sox2, Klf4 and c-Myc retroviruses.
- At day 4 after infection, the cells were reseeded into a 10 cm culture dish on feeder cells.
- At day 5 after infection, culture medium was changed to iPSC medium (DMEM/Nutrient Mixture F-12 Ham (DMEM/F12) supplemented with 20% of knockout serum replacement , 10 ng/ml bFGF , L-glutamine, and non-essential amino acids and 2-mercaptoethanol).
- Colonies, which could self-renew and expand, were picked up and reseeded onto feeder cells around day 30.
- To isolate human iPSCs and induced epithelial stem cells (iESCs), each colony was picked up and reseeded into Matrigel-coated dishes with mouse embryonic fibroblast (MEF)-conditioned iPSC medium.