Human Tumor Necrosis Factor-beta Recombinant
Categories: Recombinant Human CytokinesTNF family$70.00 – $2,800.00
Description
Accession
P01374
Source
Optimized DNA sequence encoding Human TNF beta mature chain was expressed in Escherichia Coli
Molecular weight
Native human Tumor Necrosis Factor-beta is generated by the proteolytic removal of the signal peptide and propeptide, this molecule has a calculated mass of approximately 19 kDa. Recombinant TNF-beta is a non disulfide-linked homotrimer protein,each consisting of 172 amino acids and migrates as an approximately 19 kDa protein under reducing conditions.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was Measured in a cytotoxicity assay using mouse L929 cells in the presence of the metabolic inhibitor actinomycin, and was determined to be less than 0.1ng/ml.
Protein Sequence
MTPPERLFLP RVCGTTLHLL LLGLLLVLLP GAQGLPGVGL TPSAAQTARQ HPKMHLAHST LKPAAHLIGD PSKQNSLLWR ANTDRAFLQD GFSLSNNSLL VPTSGIYFVY SQVVFSGKAY SPKATSSPLY LAHEVQLFSS QYPFHVPLLS SQKMVYPGLQ EPWLHSMYHG AAFQLTQGDQ LSTHTDGIPH LVLSPSTVFF GAFAL
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
TNF-beta is presented as a.2 μm filtered PBS solution pH7.5.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Interactor
Interactor
Q06643
Interactor
Interactor
Molecular function
Methods
Release of sST2 from placenta explants
- All placentas were from pregnant, healthy women undergoing elective caesarean section, without labor, and were processed immediately.
- Freshly delivered placentae (n = 3) were first rinsed in ice cold Hanks balanced salt solution and placed into a glove box maintained at 8% O2.
- Placental pieces, cut from undamaged lobules that appeared healthy, were rinsed in 8% O2 equilibrated ice cold explant culture medium'>medium medium'>(DMEM containing 10% foetal bovine serum , 1% antibiotic and antimycotic solution and L-glutamine) before being placed into ice cold fresh equilibrated explant culture medium'>medium.
- Placental pieces of approximately 2 mm in diameter were then dissected and distributed equally between Costar Netwell (24 mm diameter, 500 µm mesh) supports in 6-well plates containing 4 ml/well equilibrated explant culture medium (10 explants/well).
- Placental explants were finally washed again with a medium change before being incubated under conditions ‘normoxic’ for trophoblast (8% O2/87% N2/5% CO2)…
Materials
- Recombinant human TNF-α was from .
- Myosin Light Chain Kinase inhibitor PIK was generated as described
Cytokine treatment
- After 2 passages, cells were seeded into 6-well plates .
- At confluence, cytokine treatment was performed during 48 hours using human recombinant TNF-α 100 U/ml or IFN-γ 200 U/ml in serum-free media, otherwise as above.
- Experiments were ended by removal and freezing of the supernatants and addition of lysis buffer to the cell monolayer, see below.
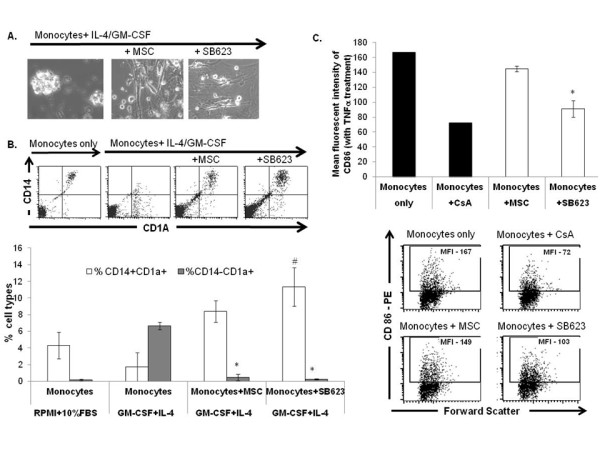
MSCs and SB623 cells interfere with monocyte-DC differentiation and maturation.
- Representative FACS data (above) and mean expression for 3 different matched lots of MSC and SB623 cells (below); the percentage of cells co-expressing CD14 and CD1A after GM-CSF+IL-4 culture is significantly higher when monocytes were cultured with either MSCs or SB623 cells versus alone (#p < 0.05); the percentage of CD14-CD1A+ cells is significantly lower when differentiation culture included either MSCs or SB623 cells versus alone (*p < 0.05); C) CD86 surface expression after 2-day TNF-α treatment; Representative FACS data (left) and mean fluorescence intensity for 3 different matched lots of MSC and SB623 cells (right); there is a significant decrease in the mean fluorescent intensity of CD86 co-stimulatory molecule when monocytes were co-cultured with SB623 cells versus parental MSCs (*p < 0.05).
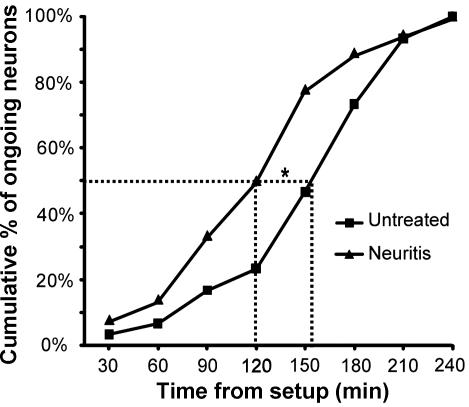
A: cumulative plot showing the time from start of the experiment, at which neurons with baseline ongoing activity were identified in the untreated group with peripheral field searches (paradigm 2; n = 30 neurons) and the neuritis group (paradigm 1; n = 36 neurons).
- B: percentage of neurons (silent and ongoing) recorded early (<151-min postsetup) and late (>151 min) in the untreated group with peripheral field searches (paradigm 2) that responded to cytokine treatment (CCL2 and TNF-α combined).
ELISA for TNFα and IL6
- Concentrations of TNF-α in supernatants were determined using the ELISA development kit supplied by .
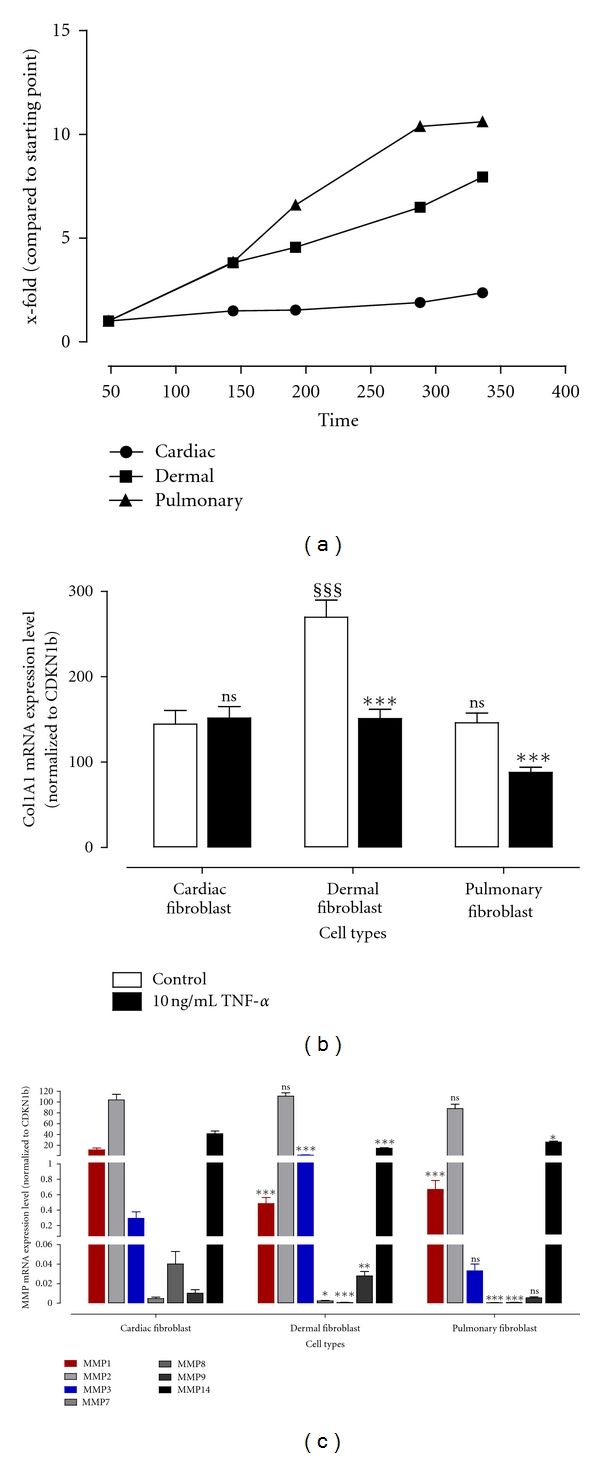
Proliferation, collagen expression, and basal MMP expression of cardiac, dermal, and pulmonary fibroblasts.
- (b) Collagen mRNA expression level was determined using TaqMan from fibroblasts incubated with or without 10 ng/mL TNF-α for 24 hours.
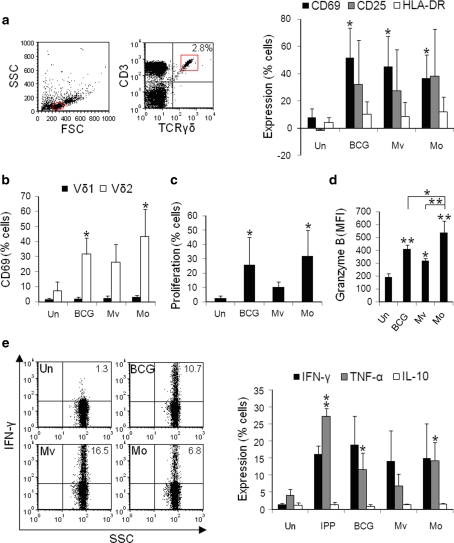
γδ T-cells within BCG-, M. vaccae- and M. obuense-treated PBMCs produce granzyme B and TH1 cytokines.
- e Percentages of γδ T-cells expressing IFN-γ, TNF-α and IL-10 were measured after 24 h of stimulation.
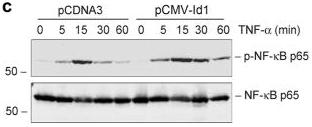
Ectopic expression of Id1 potentiates NF-κB signaling in kidney tubular epithelial cells (a, b) Id1 potentiates IκBα phosphorylation after TNF-α stimulation.
- Id1-overexpressing cells and pcDNA3-mock transfection controls were treated with TNF-α (5 ng/ml) for various periods of time.
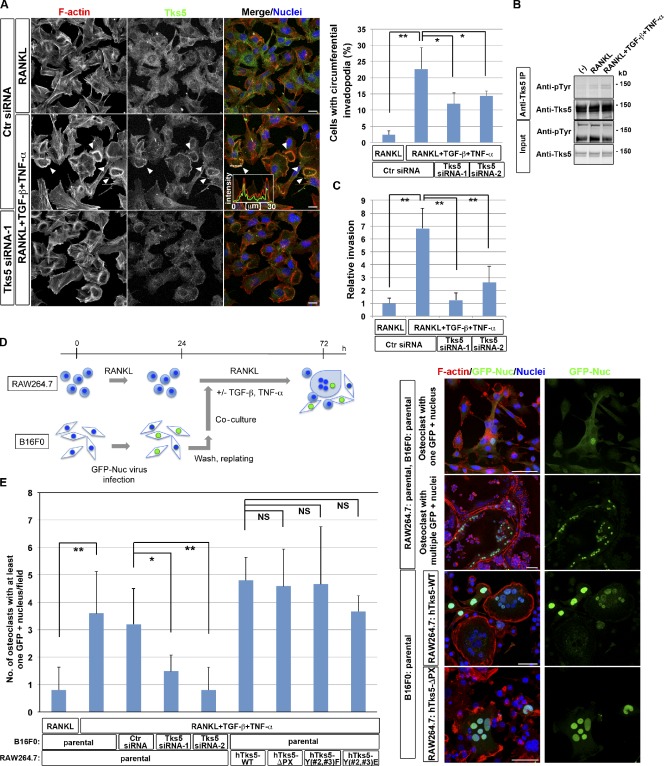
Circumferential podosomes/invadopodia mediate invasive and fusion activities.
- B16F0 murine melanoma cells transfected with control (Ctr) or Tks5 siRNAs were cultured in the presence of 10 ng/ml RANKL alone or together with 5 ng/ml TGF-β and 5 ng/ml TNF-α for 48 h. (left) The cells were then stained with rhodamine-phalloidin to visualize F-actin, with antibodies to Tks5, and with DAPI to visualize nuclei.