Human TGF alpha Recombinant
Categories: Recombinant Human CytokinesTGF-beta familyTGF-beta family$70.00 – $1,000.00
Description
Accession
P01135
Source
Optimized DNA sequence encoding Human Transforming Growth Factor Alpha mature chain was expressed in Escherichia Coli.
Molecular weight
Native human alpha, generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calculated molecular mass of approximately 26 kDa. Recombinant TGF-alpha is a disulfied linked protein consisting of 50 amino acid residue subunits. TGF-Alpha migrates as an approximately 5.5 kDa protein under reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC.
Protein Sequence
Biological Activity
The activity was determined by dose-dependent stimulation of thymidine uptake by BALB/c 3T3 cells, the ED50 was calculated at ≤ 0.2 ng/ml, corresponding to a specific activity of ≥ 5 x 10^6 units/mg.
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant TGF-A is lyophilized from 0.2 μm filtered solution with no additives.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
Upon reconstitution, this cytokine can be stored in working aliquots at 2° - 8° C for one month, or at -20° C for six months. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Q969F2
Molecular function
Molecular function
Methods
2.1. Generation and Isolation of Monocyte-Derived Langerhans Cells
- Monocyte-derived LC were generated from human peripheral blood mononuclear leukocytes (PBML) obtained from normal donors following informed consent in accordance with an , in conformity with the Declaration of .
- Blood monocytes were purified by density gradient centrifugation on Ficoll-Paque (GE healthcare, , ), followed by plastic adherence, and were cultured for 5-6 days in 6-well tissue culture plates at 2 × 106/mL in RPMI 1640 medium supplemented with 10% (v/v) FBS (PAA ), rhGM-CSF (20 ng/mL), rhIL-4 (20 ng/mL) and rh-TGF-
β (10 ng/mL) at 37°C in a humidified 5% CO2 incubator. - On day 3, fresh medium supplemented with the above mentioned cytokines was added.
- After 5 days of culture, the outcoming population consisted of typical immature LC to which half-strength concentrations of above mentioned cytokines were added.
- These LC expressed low levels of CD86, and were negative for CD83 .
- They were routinely tested for langerin and…
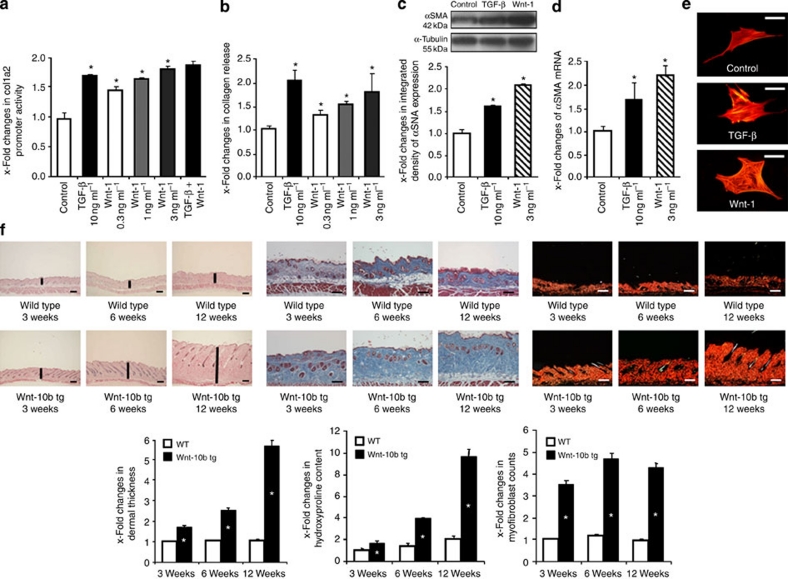
Activation of the canonical Wnt pathway induces fibrosis.
- The stimulatory effects of Wnt-1 on fibroblasts were comparable with those of TGF-β.
Mixed Lymphocyte Reaction
- Splenic naïve celltype'>celltype'>celltype'>CD4+T celltype'>cell were prepared from BALB/c with celltype'>CD4+CD62L+ T celltype'>cell isolation kit II according to manufacturer’s instructions.
- DCs were induced from C57BL/6 BM cells and pDC were sorted with a FACS Aria.
- Purified pDC were stimulated with 10 µg/ml of
L. - lactis JCM5805 or
L. - rhamnosus ATCC53103 or 0.1 µM of CpG-A overnight.
- Then, celltype'>pDC were treated with 10 µg/ml of mitomycin C for 30 min at 37oC and co-cultured with 5×105 cells/ml of BALB/c naïve celltype'>CD4+T cell in medium'>RPMI medium supplemented with or without 0.75 ng/ml of human TGF-β for 7 days.
- Cells cultured in the presence of TGF-β were collected and washed with PBS, then stained for CD3, CD4 and CD25.
- Next, cells were treated using FoxP3 staining buffer set (eBioscience)…
In vitro conversion assay
- Our conversion protocol was performed as previously reported with T cells and LpDCs obtained as described above.
- In brief, FACS-purified LpCs and C4+ T cells were co-cultured at a 1:10 ratio (1×105 C4+ T cells) in complete medium and Treg cell polarizing conditions: soluble α-C3 (1 µg/ml ) and human recombinant TGF-β (0.3 ng/ml& ).
- Co-cultures were supplemented with 5 ng/ml of recombinant human IL-2 every 2 days.
- In some experiments, CpG ODN 1555, suppressive ODN1, control ODN,
E. - coli DNA and
L. - paracasei DNA were added individually or in combination at various concentrations at the start of the co-cultures in celltype'>Treg cell polarizing conditions.
- On day 5, cells were stained with the LIVE/DEAD Fixable Blue Dead Cell Stain Kit and fluorochrome-conjugated antibodies against the cell surface markers CD4 (RM4-5) and…
Chondrogenic Differentiation
- sfMPCs were plated in triplicate (100,000 cells/well/24 well dish) and exposed to chondrogenic media for 14 days with or without micro-mass aggregation.
- Aggregation was achieved by placing 100,000 cells in a 1.5 ml sterile tube at 37°C overnight.
- Differentiation media consisted of sMPC culture media with 500 ng/mL BMP-2 , 10 ng/mL TGF-β3 , 10−8 M dexamethasone , 50 µg/mL ascorbic acid , 40 µg/mL proline , 100 µg/mL pyruvate and supplemented with insulin, transferrin, and selenium .
- Media was changed every three days during the 14-day differentiation period.
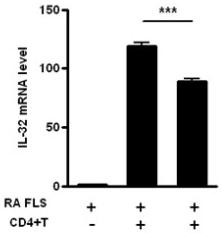
Interleukin (IL)-17/T helper (Th)17-induced IL-32 expression from rheumatoid arthritis (RA) patients.
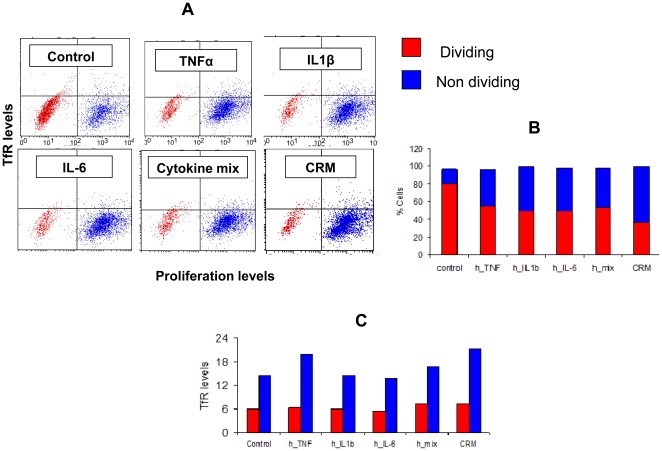
- CD4+ T cells were incubated with membrane-bound anti-CD3 antibody (2 μg/ml), IL-6 (5 ng/ml), IL1β (5 ng/ml), IL-23 (10 ng/ml), TGF-β (5 ng/ml) with or without an anti-IL-17 blocking antibody incubated for 2 h before the next incubation) for 3 days to induce Th17.polarization.
Transfection and cell treatments
- Cells were seeded in 60-mm dishes at a concentration of 2×105 for treatment with rIGF-1 or human TGF-β-1 (ocky , ).
- After serum starvation for 8 hours, CL1-5 and A549 cells were treated with 250 ng/ml rIGF-1 for 30 and 2.5 min, respectively.
- For TGF-β-1 stimulation, A549 cells were treated with 0.2 ng/ml TGF-β-1 for 5 min.
2.5. Primary splenocyte and bone marrow-derived dendritic cell (BMDC) culture
- Untreated 6-week-old SD rats were sacrificed by cervical dislocation after ether exposure.
- Cells in the spleen were harvested and prepared as a single cell suspension in complete medium in a 24-well plate (1×107 cells/well).
- In the control group, splenocytes were incubated alone or with recombinant human TGF-ß (2 ng/ml, , , ) and recombinant rat IL-6 (20 ng/ml, , , ) at 37°C for 72 h. For the experimental groups, in addition to TGF-ß and IL-6, cells were treated with culture supernatant of
F. - prausnitzii, sodium butyrate (0.05834 µmol/well), denatured
F. - prausnitzii and
B. - longum bacteria (1× 107 CFU/well).
- Each group treatment was repeated four times.
- After 72 h, the supernatant of cultured splenocytes was collected and stored at −20°C for further analysis
RNA isolation
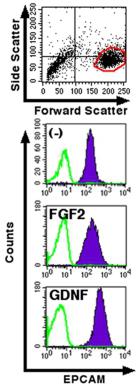
- MSCs were starved overnight in medium'>serum medium'>free medium and treated for 1 hour with recombinant human TGFα at a concentration of 10 ng/ml.
- Total RNA was extracted from untreated or TGFα-treated MSCs using TRIReagent, according to the manufacturer's protocol .
- Poly(A) RNA was isolated using theMicroPoly(A) Purist Kit .