Human SDF-1 alpha Recombinant
Categories: CXC chemokinesRecombinant Human Cytokines$70.00 – $3,500.00
Description
Accession
P48061
Source
Optimized DNA sequence encoding Human Stromal Cell-Derived Factor-1 alpha mature chain was expressed in Escherichia Coli.
Molecular weight
Native human SDF-1 alpha, generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calculated molecular mass of approximately8 kDa. Recombinant SDF-1 alpha is a monomer protein consisting of69 amino acid residue subunits, migrates as an approximately8 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
Determined by its ability to chemoattract human U937 expressing CXCR4 Determined by calcium flux with human U937 cells.
Protein Sequence
MNAKVVVVLV LVLTALCLSD GKPVSLSYRC PCRFFESHVA RANVKHLKIL NTPNCALQIV ARLKNNNRQV CIDPKLKWIQ EYLEKALNK R FKM
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant SDF-1 alpha was lyophilized from a 0.2μm filtered PBS, pH.4.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
P15172
Biological Process
Molecular function
Molecular function
Methods
Migration assay
-
Peripheral blood samples at TP2 in EDTA tubes are diluted with PBS supplemented with 2 mM EDTA and 0.5% BSA (v/v, 1∶1); 30 mL of diluted blood solution is then loaded in a 50-mL LeucoSep tubes with 15 mL of Histopaque 1077 and centrifuged at 850×
g for 25 min. - The buffy coat layers containing white blood cells are collected and washed twice with sterile PBS.
- Cells are then resuspended in EBM-2 supplemented with 0.1% BSA.
- 0.5–1×107 cells/mL of cells are loaded onto the upper part of ThinCert™ tissue culture polystyrene inserts (452.4 mm2 culture surface, 3.0 µm pore size , , ) which are pre-assembled on 6-well plates containing medium as above with or without 100 ng/mL of recombinant human SDF-1α or β-NGF , respectively.
- Cells are then incubated at 37°C in a humidified incubator with 5% CO2 for 18 hrs.
- Cells from both parts of the transwell inserts are dissociated…
Migration assay
-
Peripheral blood samples at TP2 in EDTA tubes are diluted with PBS supplemented with 2 mM EDTA and 0.5% BSA (v/v, 1∶1); 30 mL of diluted blood solution is then loaded in a 50-mL LeucoSep tubes with 15 mL of Histopaque 1077 and centrifuged at 850×
g for 25 min. - The buffy coat layers containing white blood cells are collected and washed twice with sterile PBS.
- Cells are then resuspended in EBM-2 supplemented with 0.1% BSA.
- 0.5–1×107 cells/mL of cells are loaded onto the upper part of ThinCert™ tissue culture polystyrene inserts (452.4 mm2 culture surface, 3.0 µm pore size , , ) which are pre-assembled on 6-well plates containing medium as above with or without 100 ng/mL of recombinant human SDF-1α or β-NGF , respectively.
- Cells are then incubated at 37°C in a humidified incubator with 5% CO2 for 18 hrs.
- Cells from both parts of the transwell inserts are dissociated…
Migration assay
-
Peripheral blood samples at TP2 in EDTA tubes are diluted with PBS supplemented with 2 mM EDTA and 0.5% BSA (v/v, 1∶1); 30 mL of diluted blood solution is then loaded in a 50-mL LeucoSep tubes with 15 mL of Histopaque 1077 and centrifuged at 850×
g for 25 min. - The buffy coat layers containing white blood cells are collected and washed twice with sterile PBS.
- Cells are then resuspended in EBM-2 supplemented with 0.1% BSA.
- 0.5–1×107 cells/mL of cells are loaded onto the upper part of ThinCert™ tissue culture polystyrene inserts (452.4 mm2 culture surface, 3.0 µm pore size , , ) which are pre-assembled on 6-well plates containing medium as above with or without 100 ng/mL of recombinant human SDF-1α or β-NGF , respectively.
- Cells are then incubated at 37°C in a humidified incubator with 5% CO2 for 18 hrs.
- Cells from both parts of the transwell inserts are dissociated…
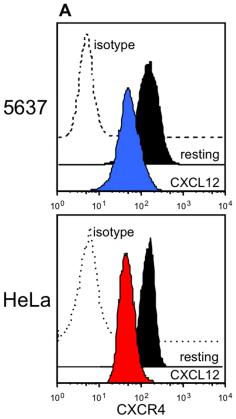
Receptors involved in the crosstalk between CXCL12 and HER1.
- 5637 or HeLa cells were cultured alone, or in the presence of 200 ng/mL CXCL12 or 25 ng/mL HB-EGF.
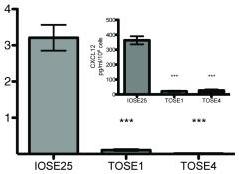
TOSE cells are hypermethylated at the CXCL12 promoter (A) CXCL12 RNA levels were measured by quantitative RT-PCR.
- CXCL12 protein levels measured by ELISA (inset).
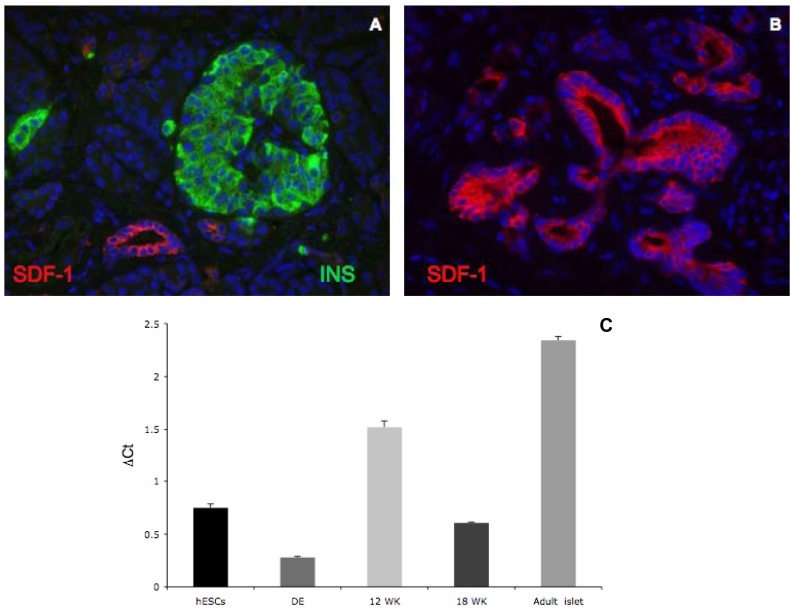
SDF-1 expression in human adult pancreas.
- Double immunofluorescent staining of SDF-1α (red) and insulin (green) in human adult islets demonstrates ductal enrichment of SDF-1α, but no expression in islets.
SDF-1 expression in human adult pancreas.
- Double immunofluorescent staining of SDF-1α (red) and insulin (green) in human adult islets demonstrates ductal enrichment of SDF-1α, but no expression in islets.
Migration assay
- Chemotaxis experiments were performed in polycarbonate transwell inserts (5 μm pore diameter .).
- Soluble SDF-1 was added in the lower chamber at a concentration of 100 ng/ml.
- Cells (2 × 105) were seeded in the upper compartment and were cultured at 37°C for 18 h. Migrated cells in the lower chamber were photographed and counted under a microscope.
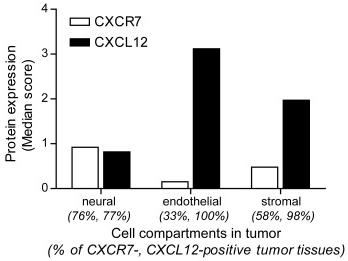
Expression of CXCR7, and its ligand CXCL12 in a NB TMA.
- Semi-quantitative assessment of CXCR7 and CXCL12 expression levels in neural, endothelial and stromal cell compartments of NB primary tumors.