Human Oncostatin M Recombinant
Categories: HematopoietinsIL-6 gp130 familyRecombinant Human Cytokines$70.00 – $4,700.00
Description
Accession
P13725
Source
Optimized DNA sequence encoding Human Oncostatin-M mature chain was expressed in Escherichia Coli.
Molecular weight
Native human Oncostatin-M is generated by the proteolytic removal of the signal peptide and propeptide, this molecule has a calculated molecular mass of approximately 26 kDa. Recombinant Oncostatin-M is a monomer protein consisting of 228 amino acid residue subunits, and migrates as an approximately 26 kDa protein under reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation off human TF-1 cells is ≤3 ng/ml, corresponding to a specific activity of ≥2 x units/mg.
Protein Sequence
MGVLLTQRTL LSLVLALLFP SMASMAAIGS CSKEYRVLLG QLQKQTDLMQ DTSRLLDPYI RIQGLDVPKL REHCRERPGA FPSEETLRGL GRRGFLQTLN ATLGCVLHRL ADLEQRLPKA QDLERSGLNI EDLEKLQMAR PNILGLRNNI YCMAQLLDNS DTAEPTKAGR GASQPPTPTP ASDAFQRKLE GCRFLHGYHR FMHSVGRVFS KWGESPNRSR RHSPHQALRK GVRRTRPSRK GKRLMTRGQL PR
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Oncostatin M was lyophilized from a 0.2μm filtered concentrated (1mg/ml) PBS solution, pH7.0.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Interactor
Biological Process
Molecular function
Molecular function
Methods
Hepatogenic differentiation
- Cells were seeded at a concentration of 5000 cells/cm2 on tissue culture plastic plates and coverslips coated with Matrigel and cultured in high glucose DMEM supplemented with 1% penicillin/streptomycin , 2 mM L-Glutamine and 10% FBS for 3 days.
- The media were then changed to high glucose DMEM supplemented with 15% FBS, 1% penicillin/streptomycin, 2 mM L-glutamine, 300 µM monothioglycerol , 20 ng/ml hepatocyte growth factor , 10 ng/ml oncostatin M , 10−7 dexamethasone , 100 ng/ml FGF4 and 1X ITS (insulin, transferrin, selenium).
- The cells were allowed to differentiate for 21 days and then fixed and stored in PBS for immunofluorescence.
- The differentiation media were collected and analysed for the presence of urea secreted by the differentiated cells.
- Urea was subsequently measured using the urea/ammonia determination kit (R-Biopharm AG, Darmstadt, Germany) according to the manufacturers instructions, with positive control being urea (as per manufacturer's instructions) and…
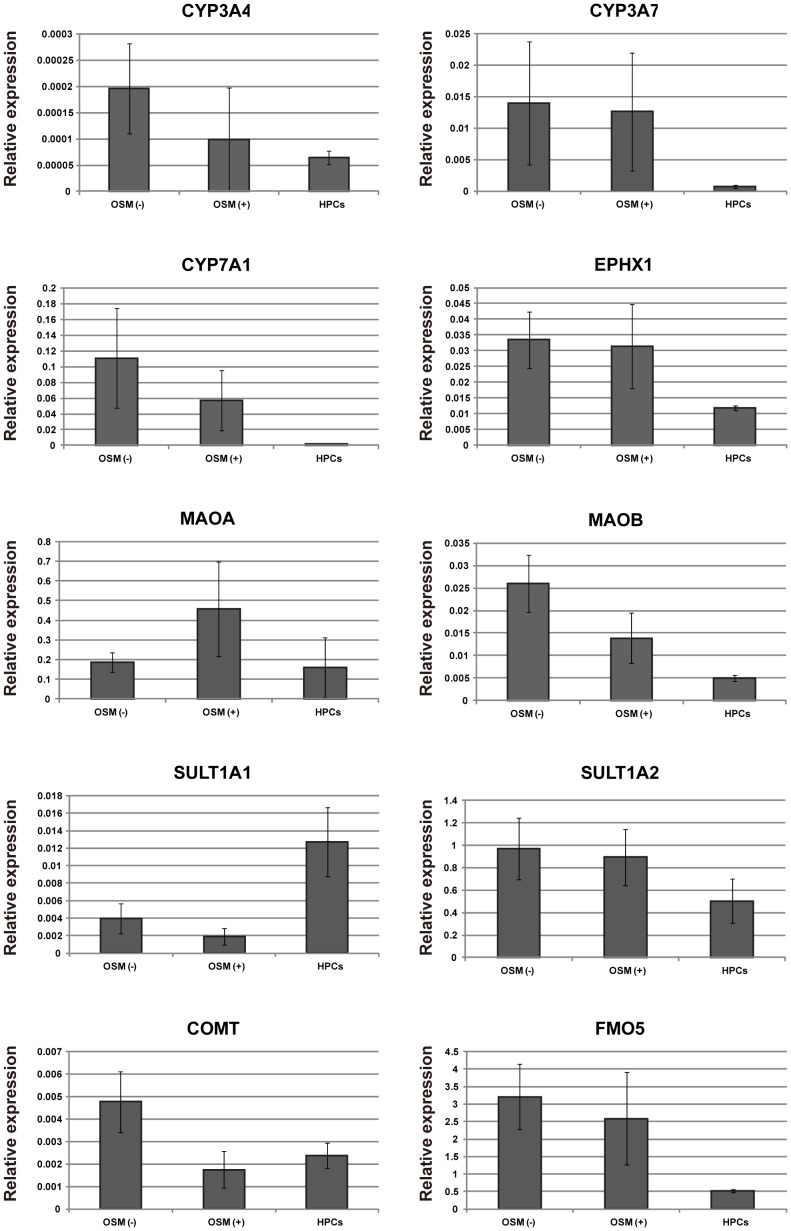
Expressions of hepatic functional genes in differentiated HPCs.
- Spheroid formation was induced by hanging drop culture in the presence or absence of OSM.
Induction of mature hepatocytic functional genes by spheroid formation
- Colonies derived from CD13highCD133+ cells were passaged and trypsinized with 0.05% trypsin-EDTA, washed in DMEM containing 10% FBS, and counted.
- The culture medium for spheroid formation was MEM supplemented with 10% FBS, 1× nonessential amino acids, 1× penicillin streptomycin glutamine, and 10−7 M dexamethasone, with or without 20 ng/ml recombinant human OSM .
- Individual drops (40 µl) containing 1×104 cells were plated on the inside of lids of 100-mm dishes containing PBS (to avoid desiccation).
- After 3 days of culture, the spheroids were collected and analyzed.