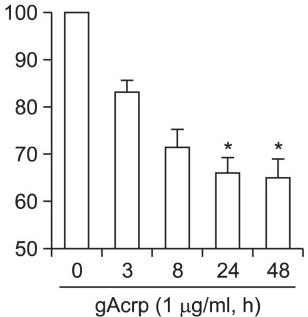
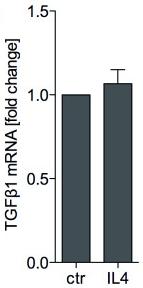
Human Noggin Recombinant
Categories: Recombinant Human CytokinesCytokines$70.00 – $3,500.00
Description
Accession
Q13253
Source
Optimized DNA sequence encoding Human Noggin mature chain was expressed in HEK293 cells.
Molecular weight
Native Human Noggin, generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calculated molecular mass of approximately 23 kDa. Recombinant Human Noggin is a disulfide-linked homodimer protein consisting of 2x205 amino acid residue subunits, migrates as an approximately 46kDa protein under non-reducingand as 23kDa under reducing conditions in SDS-PAGE.
Purity
>96%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by induced alkaline phosphatase secretion in ATDC cellsand was determined to be ≤ ng/ml, corresponding to a specific activity of ≥x units/mg.
Protein Sequence
MERCPSLGVT LYALVVVLGL RAAPAGGQHY LHIRPAPSDN LPLVDLIEHP DPIFDPKEKD LNETLLRSLL GGHYDPGFMA TSPPEDRPGG GGGPAGGAED LAELDQLLRQ RPSGAMPSEI KGLEFSEGLA QGKKQRLSKK LRRKLQMWLW SQTFCPVLYA WNDLGSRFWP RYVKVGSCFS KRSCSVPEGM VCKPSKSVHL TVLRWRCQRR GGQRCGWIPI QYPIISECKC SC
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Human Noggin was lyophilized from a 0.2 μm filtered solution in PBS, pH.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Biological Process
Biological Process
Molecular function
Methods
Growth Factors
- ecombinant human Noggin/Fc chimera and neutralizing anti-human /4 antibody from& , and from.
Growth Factors
- ecombinant human Noggin/Fc chimera and neutralizing anti-human /4 antibody from& , and from.
RA inhibits Irx1 and Irx2 expression before the onset of cell death by a BMP-independent mechanism.
- (White asterisks indicate beads soaked in BMP7 or NOGGIN).
Micromass Mesodermal Cultures
- The effect of BMP modulators and BMP2 were analyzed by adding recombinant protein to the medium in 24 hr cultures.
- Treatments were maintained for another 24 hr period.
- After testing different protein concentrations we selected the following: human recombinant BMP2 200 ngr/ml ; human recombinant NOGGIN, 200 ngr/ml ; human recombinant CHL-1, 2400 ngr/ml ; mouse recombinant CHL-2 1200 ngr/ml ; human recombinant TSG 1000 ngr/ml ; mouse recombinant AN 3000 ngr/ml ; Follistatin 800 ngr/ml .
- After these treatments we analyzed by Q-PCR changes in the expression of cartilage markers (
Sox9 ,2 ; andBmpr1b ), fibrogenic markers (, 1 and), and joint markers ( Activinβα ,Gdf5 , and). - The selected genes are well known markers of the corresponding morpho-developmental processes.
- Only
Jaws has not been used very often as joint marker, but it has been shown that it is essential for the formation of interphalangeal joints
Directed differentiation protocols
- Differentiation of hESC towards neuroectoderm used the protocol described in ref.
- 2 in N2B27 media supplemented with 10 μM Y-27632.
- Twenty-four hours later, media was changed to SN media (similar to standard hESC media, except lacking FGF2 and containing with 50 ng ml−1 human recombinant Noggin and 10 μM SB431542 .
- Cells were fixed and stained for, , and FoxA2 after 5 days in SN media.
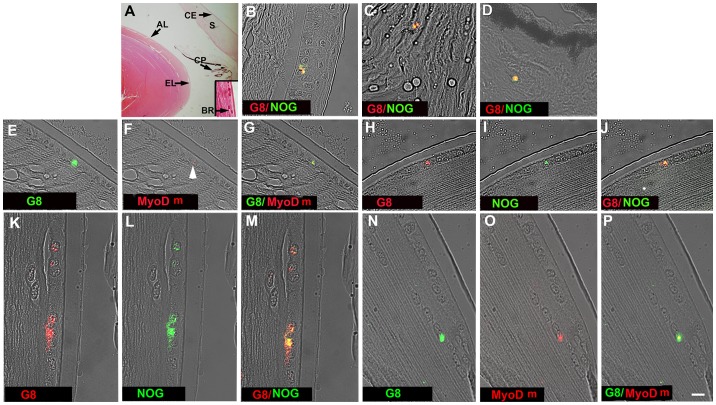
Distribution of Myo/Nog cells in the human anterior segment.
- Tissue sections through the anterior segment were stained with H&E or double labeled with G8 and dendrimers to MyoD mRNA (MyoD m) or an antibody to Noggin .
Distribution of Myo/Nog cells in the human anterior segment.
- Tissue sections through the anterior segment were stained with H&E or double labeled with G8 and dendrimers to MyoD mRNA (MyoD m) or an antibody to Noggin .
Crypt isolation, cell lines and culture conditions
- Isolation and culture of intestinal crypts were done as described previously with modifications indicated.
- Briefly, the small intestine of 4- to 12-week-old C57BL/6 wild-type or Bim-deficient mice was opened longitudinally and villi were scraped off using a coverslip.
- The intestine was cut into 1–2 cm pieces, washed three times with cold PBS and incubated with 2 mM EDTA in PBS for 30 min at 4 °C on a rotating wheel.
- Residual villi were removed by gentle shaking, the villi containing supernatant was removed and replaced with cold PBS.
- This procedure was repeated until no villi could be observed anymore in the supernatant using microscopy.
- Crypts were then detached from the basal membrane by vigorous shaking.
- The crypts enriched in the supernatant were passed through a 70-
μ m cell strainer , centrifuged at 100 ×g (3 min, 4 °C) and resuspended in 10 ml PBS for counting using microscopy. - Pelleted crypts were resuspended in Matrigel at a desired crypt density.
- Two hundred to five hundred…
Cell culture and differentiation
- IPS cells derived from FRDA patient fibroblasts (FA3 iPS and FA4 iPS) 2.
- Colonies were mechanically dissected every 7 days and transferred to freshly prepared human embryonic fibroblasts.
- The FA3-GFP cells were generated by transfecting FA3 iPS cells with the
piggyBac transposon vector (Wellcome Trust Sanger Institute) modified to contain a GFP expression cassette, driven by the human elongation factor 1 alpha promoter. - Positive GFP-expressing cells were enriched by mechanical dissection of transfected FA3 iPS cells, using a MZFIII .
- For neural induction, colonies were treated with 500 ng/ml human recombinant noggin and 4 ng/ml bFGF in neural basal media (NBM)