Human Neurotrophin-3 Recombinant
Categories: Neurotrophic factorsRecombinant Human Cytokines$70.00 – $4,700.00
Description
Accession
P20783
Source
Optimized DNA sequence encoding Human Neurotrophin-3 mature chain was expressed in Escherichia Coli.
Molecular weight
Human Neurotrophin-3 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately14 kDa. RecombinantNT-3 is a disulfide-linked homodimer protein consisting oftwoamino acid residue subunits, and migratesas an approximately27 kDa protein under non-reducing and as kDa under reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent induction of choline acetyl transferase activity in rat basal forebrain primary septal cell cultures was found to be in the range of-50 ng/ml.
Protein Sequence
MSILFYVIFL AYLRGIQGNN MDQRSLPEDS LNSLIIKLIQ ADILKNKLSK QMVDVKENYQ STLPKAEAPR EPERGGPAKS AFQPVIAMDT ELLRQQRRYN SPRVLLSDST PLEPPPLYLM EDYVGSPVVA NRTSRRKRYA EHKSHRGEYS VCDSESLWVT DKSSAIDIRG HQVTVLGEIK TGNSPVKQYF YETRCKEARP VKNGCRGIDD KHWNSQCKTS QTYVRALTSE NNKLVGWRWI RIDTSCVCAL SRKIGRT
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant NT-3was lyophilized from a 0.2 μm filtered PBS solution pH7.5.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
P07174
Interactor
Interactor
Q16288
Interactor
Q91407
Molecular function
Methods
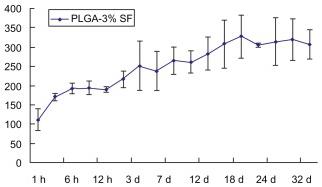
Secretion of NT-3 from NT-3-PLGA carriers loaded with three different SF solutions groups for at least 4 weeks.
- Daily release of NT-3 was examined by ELISA in the 3% SF solution group; the 1% SF solution group; the 6% SF solution group, respectively; and There was cumulative release of NT-3 across the three different groups after at least 4 weeks.
Differentiation of FRDA iPSCs into neurons and their characterisation.
- Representative images show an iPSC colony treated with noggin for 14 days, cultured as neuropheres, and neuropheres undergoing neural differentiation in the presence of neurotrophic factors, BDNF and NT3 (bright fields) and neurons (immunostained for β-tubulin III; green).
Culture and In Vitro Differentiation of Human iPSCs
- For in vitro differentiation, iPSC colonies were detached from the feeder layers en bloc using a dissociation solution (0.25% trypsin, 100 μg/ml collagenase IV [], 1 mM CaCl2, and 20% KSR; day 0) and cultured in suspension in bacteriological dishes to form EBs in a humidified atmosphere of 3% CO2.
- From day 1 to 4 of EB formation, 3 μM dorsomorphin , 3 μM SB431542 , and 3 μM BIO ((2′Z, 3′E)-6-bromoindirubin-3′-oxime) were added.
- In addition, 1 μM retinoic acid and 1 μM purmorphamine were added on days 4 and 7, respectively, and maintained thereafter until day 16 .
- The medium was changed every 2 days.
- On day 16, the EBs were enzymatically dissociated into single cells using TrypLE Select , and the dissociated cells were cultured in suspension at a density of 1 × 105 cells/ml in proliferation medium consisting of medium'>serum-free medium (media hormone mix [MHM]; 2.
- The medium was changed every 4∼6 days for…
Survival Assays
- Acutely isolated astrocytes were plated at 2,000 cells/well in poly-d-lysine-coated (PDL) 24-well plates in base media with 0.5 µg/ml aphidicolin .
- Unless otherwise noted, candidate cytokines were individually added at the following concentrations: 5 ng/ml HbEGF ; 100 ng/ml IGF2 ; 100 ng/ml CXCL12 ; 50 ng/ml BNF ; 10 ng/ml NT3 ; 10 ng/ml FGF1, , and FGF9 ; 100 ng/ml BMP2, , , , , , , GF3, and GF5 ; 250 ng/ml Activin A ; 100 ng/ml Nodal ; 100 ng/ml TGFB2 .
- Survival was assayed at 3DIV using a Live/Dead kit in which calcein AM stains live cells green and ethidium homodimer stains dead cells red .
- At least 3 independent experiments were conducted for each condition.
- For each experiment, 3 non-overlapping 20x fields per well were quantified in triplicate wells.
- Significance determined using one-way ANOVA with Dunnett correction.
In vitro differentiation of Nes-GFP+ cells
-
Neurogenic differentiation Neural differentiation of theNes -GFP+ cells was induced by plating cells onto poly-D-lysine/laminin-coated 24-well plates in medium'>N2B27 medium containing 10 ng/ml brain-derived neurotrophic factor and 10 ng/ml neurotrophin-3 , and the cells were maintained for 2 weeks. - For astroglial differentiation, the
Nes -GFP+ cells were exposed to 1% FBS and bone morphogenic protein (BMP)-4 (10 ng/ml) in medium'>N2B27 medium for 7 days. - At each experimental endpoint, the differentiated cells were identified by immunostaining using the Tuj-1 and GFAP antibodies shown in