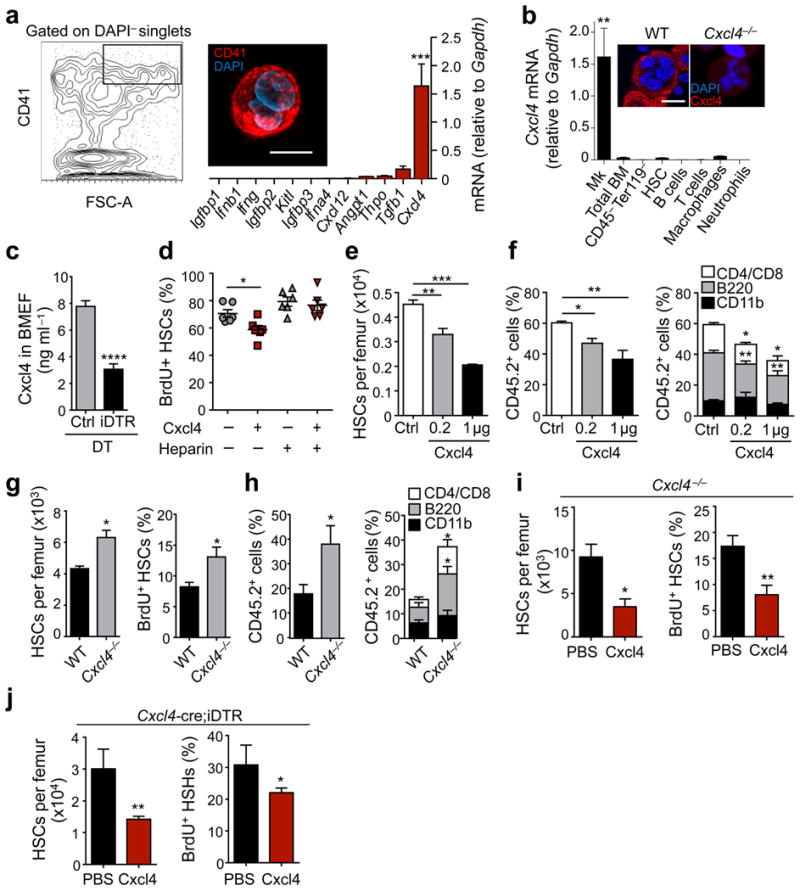
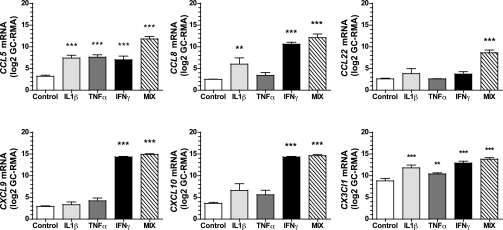
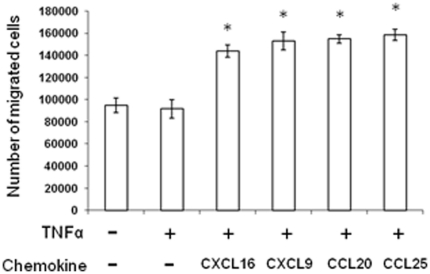
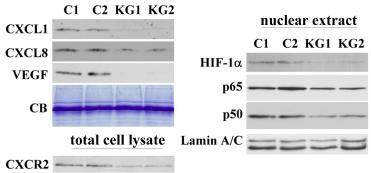
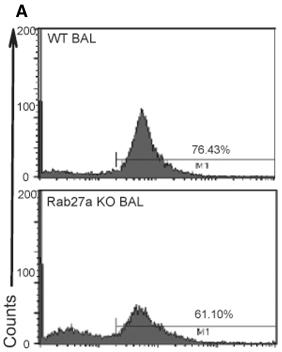
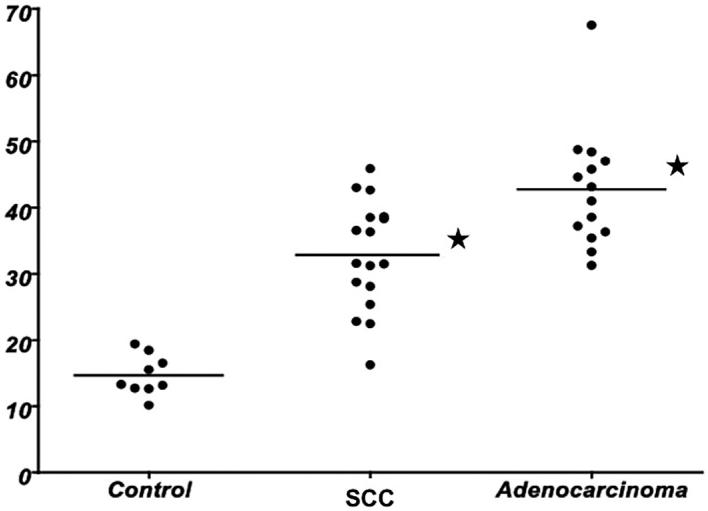
Description
Accession
P02775
Source
Optimized DNA sequence encoding HumanNAP-2 (CXCL7) mature chain was expressed in Escherichia Coli.
Molecular weight
Human NAP2, generated by the proteolytic removal of the signal peptide and propeptide and has a calculated molecular mass of approximately8 kDa. RecombinantNAP-2 is a monomeric protein consisting of amino acid residue subunits and migrates as an approximately8 kDa protein under non-reducing conditions and reducing conditions in SDS-PAGE.
Purity
>98%, as determined by SDS-PAGE and HPLC
Biological Activity
Determined by its ability to chemoattract human monocytes using a concentration range of.0-40.0 ng/ml.
Protein Sequence
MSLRLDTTPS CNSARPLHAL QVLLLLSLLL TALASSTKGQ TKRNLAKGKE ESLDSDLYAE LRCMCIKTTS GIHPKNIQSL EVIGKGTHCN QVEVIATLKD GRKICLDPDA PRIKKIVQKK LAGDESAD
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant NAP2 was lyophilized from a 0.2 μm filteredmM PB,100mM NaCl solution pH.5.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
P25024
Interactor
P25025
Biological Process
Molecular function
Molecular function
Molecular function
Methods
Cell culture
- Human THP-1 monocytic cells were subcultured in RPMI 1640 as described previously in detail 6 cells/ml in RPMI 1640 supplemented with 10% FBS and 5 µg/ml gentamicin.
- After 24 h, 1 or 10 µg/ml globular adiponectin was added and the cells were incubated for 6 to 24 h. Globular adiponectin is a recombinant protein derived from human globular domain adiponectin cDNA expressed in
Escherichia coli . - This protein was endotoxin free (<2 EU/µg) according to the manufacturer.
- In addition, treatment of cells with globular adiponectin (10 µg/ml) in the presence of polymyxin B (50 µg/ml) did not affect globular adiponectin-related
TNFα expression (data not shown). - The ox-LDL incubation experiments were performed like previously described 6 cells/ml in glucose-free RPMI 1640 supplemented with 10% FBS, 5 µg/ml gentamicin, and 5.5 mM D-glucose in a 5% CO2 incubator at 37°C.
- After 24 h, 9.5 mM D-glucose…
Cell culture
- Human THP-1 monocytic cells were subcultured in RPMI 1640 as described previously in detail 6 cells/ml in RPMI 1640 supplemented with 10% FBS and 5 µg/ml gentamicin.
- After 24 h, 1 or 10 µg/ml globular adiponectin was added and the cells were incubated for 6 to 24 h. Globular adiponectin is a recombinant protein derived from human globular domain adiponectin cDNA expressed in
Escherichia coli . - This protein was endotoxin free (<2 EU/µg) according to the manufacturer.
- For glucose incubation experiments, cells were cultured at a density of 1×106 cells/ml in glucose-free RPMI 1640 supplemented with 10% FBS, 5 µg/ml gentamicin, and 5.5 mM D-glucose in a 5% CO2 incubator at 37°C.
- After 24 h, 9.5 mM D-glucose or 9.5 mM D-mannitol (osmotic control) was added and incubated for 24 h under normal growth conditions.
- Cell viability, as determined by trypan blue exclusion, was…
Growth Factors
- ecombinant human Noggin/Fc chimera and neutralizing anti-human /4 antibody from& , and from.
Crypt isolation and organoid culture
- Organ culture of freshly isolated human small intestinal biopsies was performed in medium'>RPMI medium .
- For organoid culture, crypts were isolated from the small intestine of mice and cultured for a minimum of 7 days as previously described.
- In brief, crypts were isolated by incubating pieces of small intestine in isolation buffer (phosphate buffered saline without calcium and magnesium (PBSO), 2 mM EDTA).
- Crypts were then transferred into matrigel in 48 well plates and 350 μl culture medium (Advanced ME/F12 , containing HEPES (10 mM, PAA), GlutaMax (2 mM), Penicillin (100 U/ml), Streptomycin (100 μg/ml), murine EGF (50 ng/ml), recombinant human-spondin (1 μg/ml& ), N2 Supplement 1x , B27 Supplement 1x , 1 mM N-acetylcystein and recombinant murine Noggin (100 ng/ml)).
- Organoid growth was monitored by light microscopy.
- In some experiments, human biopsies or organoids were treated with recombinant mouse TNF-α (25 ng/ml), recombinant human TNF-α (50 ng/ml),…
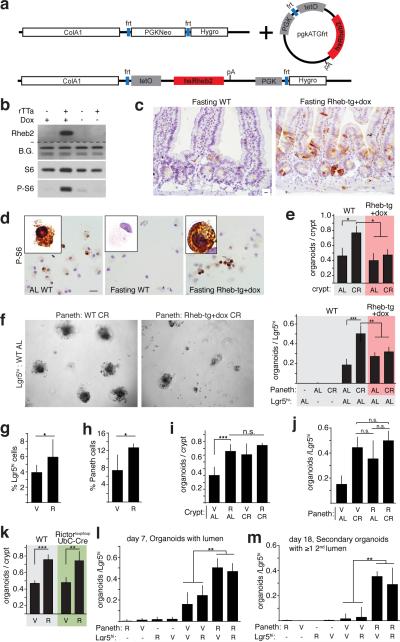
mTORC1 signaling in Paneth cells mediates the effects of calorie restriction on ISC function a. Schematic of the Tet-ON human Rheb2 transgene (Rheb-tg).
- Organoid potential of intestinal crypts (n=3) (i) and AL Lgr5hi ISCs with Paneth cells (n=5) (j) from R, CR, or CR+R treated mice.
Cell culture
-
DCs were generated from mouse spleens as described elsewhere
Swap-70 andSwap-70 mice were homogenized through a cell strainer. - After red blood cell lysis, cells were washed once and seeded at a density of 1×106 cells/ml in MEM supplemented with 10% fetal calf serum (FCS) , 1% Penicillin/Streptomycin (10000 U/ml/10 mg/ml ), 50 µM 2-mercaptoethanol, 10% GM-CSF supernatant obtained from J558 cells, and 1 ng/ml recombinant human TGF-β (& or) into a 24 well plate (1 ml/well) at 37°C in a humidified atmosphere and 5% CO2.
- Every 4 to 5 days half of the medium was changed.
- DCs were used at days 12 to 16 of culture at which point 80 to 90% of the cells were CD11c positive as analyzed by FACS (data not shown).
Crypt isolation, Enteroid Culture and Analysis
- The pelleted epithelium was re-suspended directly into hESC Matrigel supplemented with 100 ngmL−1 recombinant mouse Noggin , 500 ngmL−1 recombinant human-Spondin , 50ngmL−1 recombinant mouse EGF , 100 ngmL−1 recombinant human Wnt3a (& systems), 10 µM Y-27632 , 10 µM SB202190 , and 500 nM LY2157299 .
- Between 50 and 200 crypt/villi units were plated in 50 uL of matrigel on a 48 well plate.
- After allowing the matrix to polymerize for 30 min at 37°C, each well was overlaid with 500 µL of Advanced DMEM/F12 containing the supplements 1× N-2 supplement , 1× B-27 supplement minus vitamin A , 1× Glutamax , 100 µgmL−1 penicillin/streptomycin, 1 mM Hepes buffer , and 1 mM N-Acetylcysteine.
- Growth factors were added to the media 48 hr after plating and every 72 hr following that.
- The entire volume of media was changed 72 hr following plating and every 72 hr after that.
- Every 1–2 weeks organoids…
Induction of cholangiocytic cyst formation by human iPS cell-derived CD13highCD133+ cells
- Colonies derived from CD13highCD133+ cells were passaged and trypsinized using 0.05% trypsin-EDTA, washed in DMEM containing 10% FBS, and then counted.
- The cells were then combined with an extracellular matrix gel consisting of a mixture of 40% collagen type-I and 40% Matrigel , and cultured in 24-well culture plates (1500 cells/50 µl extracellular matrix gel/well).
- After the 10 min incubation, culture medium was added, followed by incubation for 10–12 days with medium changes every 3 days.
- The culture medium was a 1∶1 mixture of medium'>H-CFU-C medium and MEM/F-12 supplemented with 2% B27 supplement, 0.25 µM A-83-01, 10 µM Y-27632, 20 ng/ml EGF, 40 ng/ml HGF, 40 ng/ml recombinant human Wnt-3a , and 100 ng/ml recombinant human-spondin 1 .
- Cysts in gels were stained according to previously described methods
Colony forming analysis
- celltype'>Leukemic cell lines, MNCs from the patients with, or healthy controls were infected with or without the indicated viruses (50 MOI) and seeded in triplicate in a mixture containing 1.35% methylcellulose in medium'>Iscove's medium'>modified medium'>Dulbecco's medium (IMDM;) supplemented with 20% fetal bovine serum and10−4 M 2-Mercaptoethanol .
- For colony assay of bone marrow cells, 3 U/mL recombinant human (rh) erythropoietin, 50 ng/mL rh stem cell factor, 30 ng/mL rh granulocyte macrophage–colony-stimulating factor, and 10 ng/mL rh interleukin-3 were added to the methylcellulose medium.
- The colonies were evaluated under a microscope on day 12 of culture.
- In contrast, leukemic cell lines were seeded in cytokine-free mixture as described previously [
Induced pluripotent stem cell-directed differentiation
- For hepatocyte differentiation, post-excised iPSCs were grown on Matrigel as stated above until reaching a 60 to 70% confluence upon which endoderm induction was initiated by replacing the post-excised iPSCs for 24 hours with RPMI 1640 medium , supplemented with 0.5 mg/ml albumin fraction V , and 100 ng/ml Activin A .
- On the following 2 days, 0.1 and 1% insulin–transferrin–selenium were added to the medium, respectively.
- Post-excised iPSCs were then cultured in hepatocyte culture medium containing 30 ng/ml fibroblast growth factor-4 and 20 ng/ml BMP2 for 4 days.
- The now-differentiated cells were then incubated in hepatocyte culture medium'>medium containing 20 ng/ml hematopoietic growth factor and 20 ng/ml keratinocyte growth factor for 6 days, in hepatocyte culture medium'>medium containing 10 ng/ml oncostatin-M plus 0.1 μM dexamethasone…