Human Interleukin-8 (72AA) Recombinant
Categories: CXC chemokinesRecombinant Human Cytokines$70.00 – $2,700.00
Description
Accession
P10145
Source
Optimized DNA sequence encoding Human Interleukin-8 mature chain was expressed in Escherichia Coli.
Molecular weight
Native human IL-8, generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calculated molecular mass of approximately 8 kDa. Recombinant IL-8 is a monomer protein consisting of 72 amino acid residue subunits, and migrates as an approximately 8 kDa protein under reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
Determined by its ability to chemoattract human peripheral blood neutrophils using a concentration range of.0-100.0 ng/ml.
Protein Sequence
MTSKLAVALL AAFLISAALC EGAVLPRSAK ELRCQCIKTY SKPFHPKFIK ELRVIESGPH CANTEIIVKL SDGRELCLDP KENWVQRVVE KFLKRAENS
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than.1 ng/µg(1EU/µg).
Presentation
Recombinant IL-8 was lyophilized from a.2 μm filtered PBS solution pH7.5 .
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than.1 mg/mL. This solution can then be diluted into other buffers
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at° -° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
P25024
Interactor
P25025
Interactor
Q16570
Biological Process
Biological Process
Molecular function
Methods
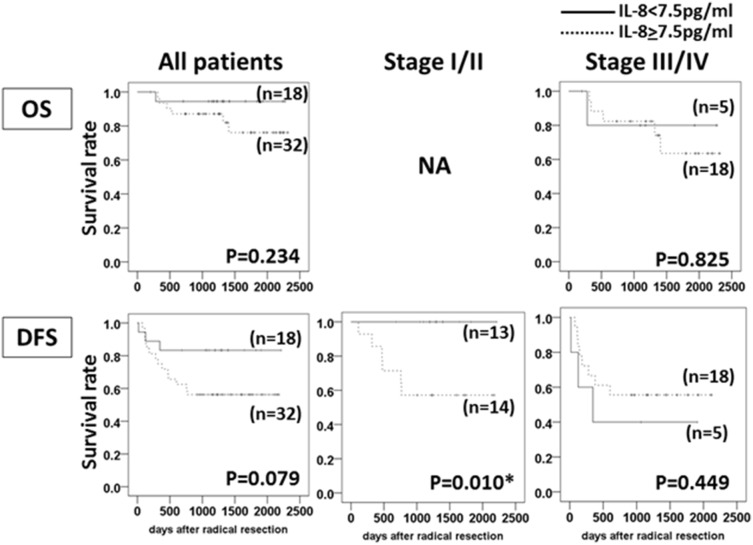
The relationship between serum IL-8 levels and the clinical outcome in OSCC patients who underwent radical resection of their tumor(s).
- The differences in the serum IL-8≥7.5 pg/ml vs. IL-8<7.0 pg/ml in all patients, in the Stage I/II patients and in the Stage III/IV patients were calculated by log-rank test.
Flow chamber experiments
- Flow chamber experiments were conducted as described [6 cells/ml) for leukocyte staining.
- The number of neutrophils was now set at 2×106 cells/ml by counting fluorescent cells in a Neubauer chamber by fluorescent microscopy using the FITC channel (BX51 WI , with a saline immersion objective × 20/0.95 NA, Olympus Hamburg).
- The cell suspension was perfused through the flow chamber and neutrophil adhesion was observed by fluorescent microscopy for 10 minutes under constant flow conditions using a high precision perfusion pump ( , , wall shear stress 0.1).
- Images were recorded via a CCD camera system (CF8HS; Kappa) on a Panasonic S-VHS recorder.
- Permanent adherent fluorescent cells were counted as neutrophil adhesion per field of view (FOV) after 10 min.
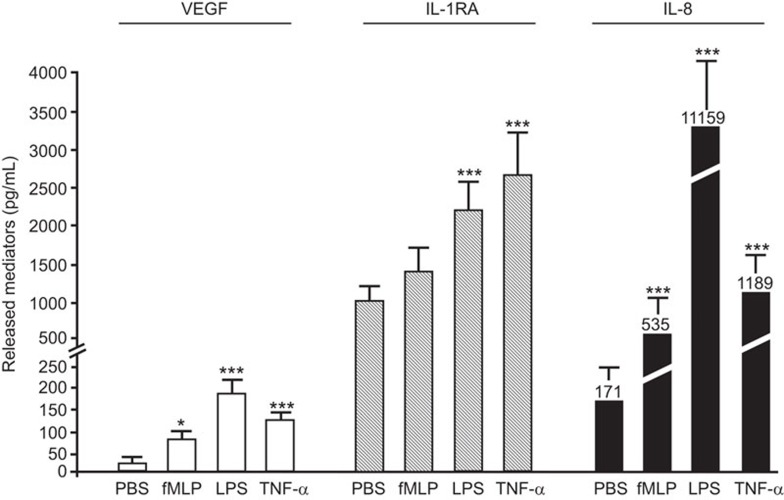
Effect of pro-inflammatory agonists on the release of inflammatory cytokines by neutrophils.
- Neutrophils were incubated for 2 and 24 h in presence of PBS (vehicle), fMLP (10−7 M), LPS (1 µg/ml) or TNF-α (10 ng/ml) for the subsequent quantification of VEGF, IL-1RA (2 h) and IL-8 (24 h) release by ELISA.