Human Interleukin-7 Recombinant (E.Coli)
Categories: HematopoietinsIL-2 familyRecombinant Human Cytokines$70.00 – $4,700.00
Description
Accession
P13232
Source
Optimized DNA sequence encoding Human Interleukin-7 mature chain was expressed in Escherichia Coli.
Molecular weight
Native human Interleukin-7 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately17 kDa. Recombinant IL-7 is a monomeric protein consisting of153 amino acid residue subunits, and migrates as an approximately17 kDa protein under non-reducing conditions and reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) determinedby dose-dependent stimulation of the proliferation of murineE8 cells is ≤.5 ng/ml, corresponding to a specific activity of ≥ x units/mg.
Protein Sequence
MFHVSFRYIF GLPPLILVLL PVASSDCDIE GKDGKQYESV LMVSIDQLLD SMKEIGSNCL NNEFNFFKRH ICDANKEGMF LFRAARKLRQ FLKMNSTGDF DLHLLKVSEG TTILLNCTGQ VKGRKPAALG EAQPTKSLEE NKSLKEQKKL NDLCFLKRLL QEIKTCWNKI LMGTKEH
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Interleukin-7 was lyophilized from a 0.2 μm filtered PBS solution pH7.5.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Molecular function
Molecular function
Methods
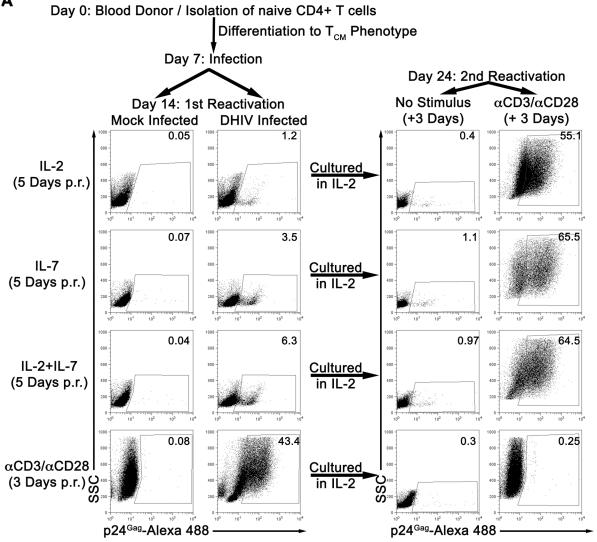
IL-7 induces partial reactivation of latent HIV-1 in cultured TCM.
- Mock or DHIV latently infected, cultured TCM were incubated in the presence of IL-2, IL-7 or a combination of IL-2 and IL-7 (IL-2+IL-7) or costimulated with antibodies to CD3 and CD28 (αCD3/αCD28) and assessed for intracellular p24Gag by flow cytometry (1st Reactivation).
Splenic Treg and Tcon cells display different susceptibility to mitochondrial apoptosis.
- Cells were cultured either in medium for 9, 18 and 48 h or in the presence of 100 U/ml IL-2, 20 ng/ml IL-7, 1 μM SAHA, 100 ng/ml FasL, 10−8 M Dexamethasone, 10 μg/ml Etoposide and 100 nM Staurosporine for 18 h. For calculation of increased and relative survival cell viability was normalized to medium values.
Cell culture
- Sorted ILC subsets were cultured overnight in the presence of IL-7 and SCF R&D Systems; both at 10 ng/ml) in the presence or absence of PMA (50 ng/ml) and Ionomycin (100 ng/ml) or for 3 days with IL-7, SCF, and either IL-23 or IL-1b.
- Cells were cultured in 200 μl DMEM supplemented with 10% FCS .
- IL-17a and IL-22 production was measured using the uoSet ELISA development kit (&systems).
- ELISAs were performed according to the manufacturers’ instructions.
Cell culture
- Sorted ILC subsets were cultured overnight in the presence of IL-7 and SCF R&D Systems; both at 10 ng/ml) in the presence or absence of PMA (50 ng/ml) and Ionomycin (100 ng/ml) or for 3 days with IL-7, SCF, and either IL-23 or IL-1b.
- Cells were cultured in 200 μl DMEM supplemented with 10% FCS .
- IL-17a and IL-22 production was measured using the uoSet ELISA development kit (&systems).
- ELISAs were performed according to the manufacturers’ instructions.
Priming assays
- Cytotoxic T-lymphocytes were generated by loading Mel888 or MV-treated Mel888 onto DC at a ratio of 3:1.
- After 24 hours non-adherent C were harvested then cocultured with autologous PBMC at ratios varying from 1:15 to 1:45 in CTL media (PMI supplemented with 7.5% (v/v) human AB serum ( , ’s , ), 1% (v/v) L-glutamine, 1% v/v sodium pyruvate , 1% (v/v) non-essential amino acids, 1% (v/v) HEPES , 20μM β-mercaptoethanol and 5ng/ml IL-7 .
- IL-2 was added at 30IU/ml on day 4 only.
- CTL were restimulated after one week in an identical fashion.
Generation of CD8 T-cell clones.
- CD8 T-cell clones specific for PPI3–11 were generated as described (3–11 peptide in X-Vivo/5% AB serum for 1 h at 37°C.
- After extensive washing, matured DCs were cultured at a 1:30 ratio with freshly obtained immunomagnetically isolated CD8 T cells (CD8 Isolation Kit II ) in 48-well culture plates in X-vivo/5% AB serum supplemented with 10 ng/mL IL-7 , 0.1 ng/mL IL-15, 5% Cellkine , and 25 IU/mL IL-2 .
- On day 10, cells were harvested and stained with anti-CD3 (V500), anti-CD8 (APC H7), violet amine reactive dye (live/dead exclusion; pacific blue), and PPI3–11 tetramer (PE and APC) as appropriate.
- CD3+CD8 double tetramer-positive T cells were flow sorted (for gating strategy, see
Supplementary. - 1) and seeded at one cell per well into 96-well plates containing 0.2 × 106 irradiated PBMCs in culture medium (CM; X-Vivo/5% AB serum, with IL-7 [10 ng/mL], IL-15 [0.1 ng/mL], IL-2 [100 IU/mL], and 2.5%…
Mixed lymphocytes tumor cell culture (MLTC)
- PBMCs isolated from melanoma patients were thawed and incubated overnight at 37°C.
- PBMCs (106 cells/well) were cultured in the presence of autologous irradiated (150 Gy) melanoma cells at a lymphocyte to tumor ratio of 5:1 in 24-well plates with X-VIVO15 and 5% HS.
- Different cytokine combinations were added to these MLTCs in order to select the most suitable in vitro culture conditions in terms of T lymphocyte expansion and efficiency in tumor cell recognition: (1) 120 IU/ml rhIL-2 ; (2) 120 IU/ml rhIL-2 and 10 ng/ml rhIL-15 ; (3) 10 ng/ml rhIL-15; (4) 5 ng/ml rh-IL-7 ; (5) 120 IU/ml rhIL-2 and 5 ng/ml rhIL-7; (6) 120 IU/ml rhIL-2 and 10 ng/ml rhIL-21 ; (7) 10 ng/ml rhIL-21 alone.
- Fresh medium containing the above indicated cytokines was replaced every 3 days.
- These MLTCs were stimulated weekly with irradiated autologous melanoma cells, and their reactivity was tested starting from the 3rd week of culture.
CTL Induction
- Naïve CD8+ T cells were negatively isolated from PBMCs of A24-positive donors using the CD8+ T cell isolation kit II and anti-CD45RO microbeads .
- The isolated cells were more than 95% pure CD45RO− CD8+ populations.
- These CD8+ T cells (1×106 cells/well) were cocultured with irradiated (100 Gy) aAPCs (1×105 cells/well) in 2 mL X-VIVO20 supplemented with 5% human AB serum (MP, , ) in wells of a 12-well plate in the presence of 10 ng/mL IL-12 .
- On day 3, 10 ng/mL IL-7 and IL-15 were added.
- Every 3 days, half the medium was exchanged for fresh medium containing 10 ng/mL IL-15.
- On day 12, the T cells were restimulated with γ-irradiated aAPCs.
- One day thereafter, IL-2 was added, to a final concentration of 20 U/mL.
- celltype'>To establish celltype'>T cell clones, a limiting dilution of the polyclonal Ccelltype'>TL was performed as…
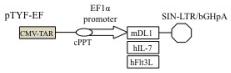
Enhanced proliferation of T cell precursors on LmDL1-FL7., Lentivector constructs expressing mouse DL1, human IL-7 and human Flt3L.
- , qRT-PCR analysis for DL-1, Flt3L, and IL-7.
Hematopoietic Stem/progenitor Cell Cultures
- Stromal cells were irradiated (2000 rad) and plated (25,000 cells/cm2) on tissue culture treated wells in stroma media (see above) 24 hours before co-culture.
- HSPC were co-cultured with stroma (1000–5000 CD34+ cells/cm2) in “HSC media” (stroma media supplemented with human SCF (25 ng/ml), FLT-3L (25 ng/ml) and TPO (25 ng/ml) for 7–14 days before replating (100,000–200,000 cells/cm2).
- Half the media was replaced every other day.
- For B-cell differentiation, the expanded cells were co-cultured on irradiated OP9M2 in stroma media supplemented with human SCF (50 ng/ml), FLT-3L (40 ng/ml) and IL-7 (100 ng/ml) .
- Following expansion, cells were assayed by FACS and colony-forming assays (see below).