Human Interleukin-6 Recombinant (E.coli)
Categories: HematopoietinsIL-6 gp130 familyRecombinant Human Cytokines$70.00 – $2,700.00
Description
Accession
P05231
Source
Optimized DNA sequence encoding Human Interleukin-6 mature chain was expressed in E.Coli
Molecular weight
Native human Interleukin-6 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 21 kDa. Recombinant IL-6 is a homodimer protein consisting of 184 amino acid residue subunits, and migrates as an approximately 21 kDa protein under reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation ofmurineTD1 cells was found to be less than.1 ng/ml, corresponding to specific activity ofx107 IU/mg.
Protein Sequence
MNSFSTSAFG PVAFSLGLLL VLPAAFPAPV PPGEDSKDVA APHRQPLTSS ERIDKQIRYI LDGISALRKE TCNKSNMCES SKEALAENNL NLPKMAEKDG CFQSGFNEET CLVKIITGLL EFEVYLEYLQ NRFESSEEQA RAVQMSTKVL IQFLQKKAKN LDAITTPDPT TNASLLTKLQ AQNQWLQDMT THLILRSFKE FLQSSLRALR QM
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than.1 ng/µg(1EU/µg).
Presentation
Interleukin-6 was lyophilized from a.2 μm filtered PBS solution pH7.0.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than.1 mg/mL. This solution can then be diluted into other buffers
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at° -° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Interactor
Interactor
Interactor
Biological Process
Molecular function
Molecular function
Methods
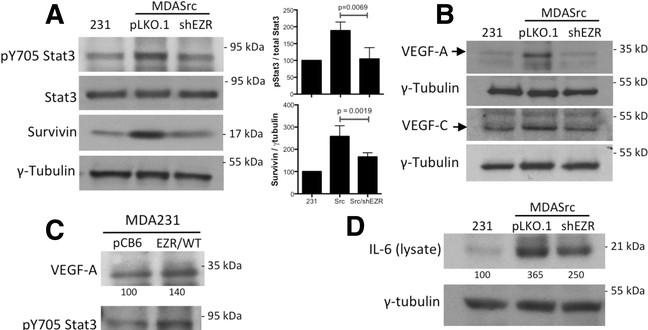
Ezrin KD blocks Stat3 phosphorylation, VEGF-A/-C, and IL-6 expression.
- Immunoblot analysis of IL-6 in cell lysates from MDA231 and MDASrc cells expressing the empty vector pLKO.1 or shEZR vector.
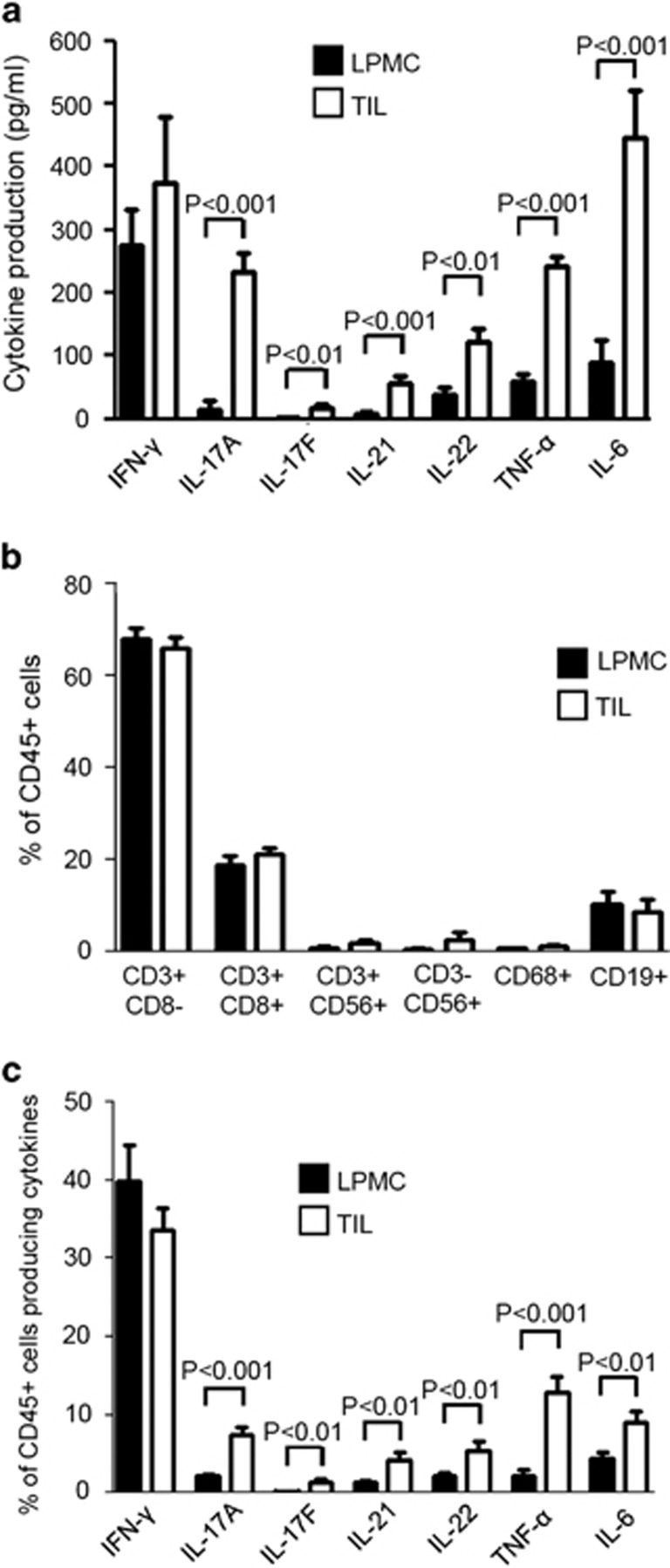
The neoplastic area of CRC samples is massively infiltrated with Th17-related cytokine-, TNF-α- and IL-6-producing cells.
- (a) IFN-γ, IL-17A, IL-17F, IL-21, IL-22, TNF-α and IL-6 proteins were analyzed by enzyme-linked immunosorbent assay in LPMC-derived supernatants (LPMC SNs) and TIL-derived supernatants (TL SNs), and data are expressed as pg/ml supernatants.
Isolation and transduction of primary murine stem- and progenitor cells
- Hematopoietic stem- and progenitor cells (negative for the mature lineage markers Mac1, Gr1, CD3, CD4, CD8, B220, Ter119, and positive for the primitive surface markers c-Kit and Sca1; LKS +) were FACS-sorted from the bone marrow of C57Bl6 wild-type mice and pre-stimulated with 50 ng/ml murine Stem Cell Factor (mSCF, , ), 10 ng/ml murine Interleukin 3 (mIL-3), and 50 ng/ml human IL-6 at 37 °C, 5% CO2 for 48 h to induce cycling.
- Pre-stimulated cells were subsequently cultured in the above described cytokines with the addition of 8 µg/ml Polybrene on retronectin-coated plates pre-loaded with viral supernatant (1–2 hits), and GFP+ cells were isolated after an additional 48 h (n = 6 independent transductions).
Selection of affibody molecules from a naïve synthetic library
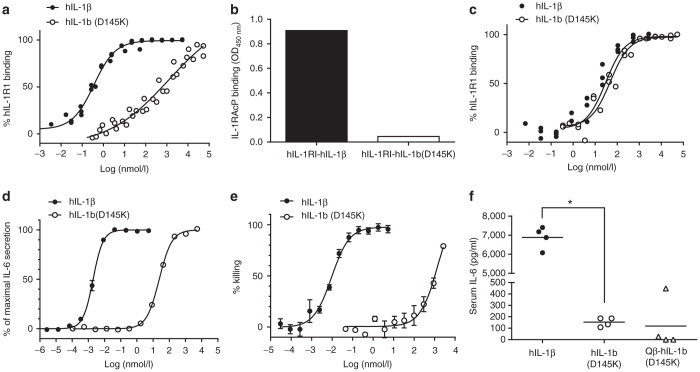
- Human IL-6 (hIL-6), was biotinylated using No-Weigh Sulfo-NHS-LC-Biotin and dialyzed to remove unbound biotin.
- A combinatorial library of affibody molecules displayed on bacteriophage was subjected to four rounds of selection using biotinylated hIL-6 (bio-hIL-6) essentially according to Grönwall et al.
Table S1 ). - The DNA sequences of the enriched clones were determined via PCR amplification of insert sequences and using an ABI PRISM® 3130xl Genetic Analyzer instrument (PE Applied Biosystems) according to the manufacturer's instructions.