Human Interleukin-6 Recombinant (E.coli)
Categories: HematopoietinsIL-6 gp130 familyRecombinant Human Cytokines$70.00 – $2,700.00
Description
Accession
P05231
Source
Optimized DNA sequence encoding Human Interleukin-6 mature chain was expressed in E.Coli
Molecular weight
Native human Interleukin-6 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 21 kDa. Recombinant IL-6 is a homodimer protein consisting of 184 amino acid residue subunits, and migrates as an approximately 21 kDa protein under reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation ofmurineTD1 cells was found to be less than 0.1 ng/ml, corresponding to specific activity of 1x107 IU/mg.
Protein Sequence
MNSFSTSAFG PVAFSLGLLL VLPAAFPAPV PPGEDSKDVA APHRQPLTSS ERIDKQIRYI LDGISALRKE TCNKSNMCES SKEALAENNL NLPKMAEKDG CFQSGFNEET CLVKIITGLL EFEVYLEYLQ NRFESSEEQA RAVQMSTKVL IQFLQKKAKN LDAITTPDPT TNASLLTKLQ AQNQWLQDMT THLILRSFKE FLQSSLRALR QM
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Interleukin-6 was lyophilized from a 0.2 μm filtered PBS solution pH7.0.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Interactor
Interactor
Interactor
Biological Process
Molecular function
Molecular function
Methods
Chemotaxis assay
- A migration assay was performed in 48- or 96-well Corning Costar transwell chambers with porous polycarbonate membranes with a pore size of 5 um .
- hMSC at passage 4 were resuspended at 1 × 106/mL in the migration medium supplemented with 1% BSA and insulin transferrin selenium (ITS) and seeded in the upper chamber.
- The following human recombinant proteins were used as chemoattractants in the lower compartment: hepatocyte growth factor (HGF), platelet-derived growth factor-AB (PDGF-AB), epidermal growth factor (EGF), VEGF-121, basic fibroblast growth factor (bFGF), insulin-like growth factor (IGF-1), macrophage inflammatory protein-3β (MIP-3β), macrophage inflammatory protein-1α (MIP-1α), B cell attracting chemokine-1 (BCA-1), regulation upon activation normal T cell express sequence (RANTES), growth regulated proteinα (GROα), fractalkine, SDF-1α, IL-1β, IL-6, IL-8, TNF-α .
- The concentration of each cytokine was optimized to ensure maximal cell migration through the porous membrane.
- The concentrations were: 40 ng/mL HGF, 10 ng/mL PDGF-AB, 10 ng/mL EGF, 10 ng/mL VEGF-121,…
Th17 cell differentiation/polarization
- Spleens of EAE mice were aseptically harvested at 19 dpi.
- Single-cell suspensions of splenocytes were prepared by pushing spleens through a sterile 70-μm pore size nylon mesh.
- CD4+CD62L+ T cells were isolated using MACS beads according to the manufacturer's protocol .
- CD4+CD62+ T cells were stimulated with plate-bound anti-CD3 antibodies (2 μg/ml; eBioscience) and soluble anti-CD28 antibodies (5 μg/ml; eBioscience) for 4 d in RPMI 1640 medium supplemented with 2 mM sodium pyruvate, L-glutamine, 10% FBS.
- T cells were polarized with human TGF-β (5 ng/ml), IL-6 (20 ng/ml), IL-23 (10 ng/ml) plus anti–IFN-γ (10 μg/ml , , ) and anti-IL-4 (10 μg/ml ) to stimulate Th17 differentiation.
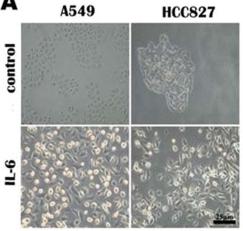
IL-6 promotes lung carcinoma cell invasion and EMT.
- A549 and HCC827 cell mesenchymal phenotype induced by IL-6 (200×).
Ethics statement, cell culture and T cell polarization
- Mononuclear cells from blood were isolated by density gradient centrifugation from healthy donors after obtaining informed, written consent in accordance with the Declarations of Helsinki principles for research involving human objects.
- The study was approved by The of for the Capital Region in (H-3-2009-132).
- Naïve CD4+ T cells were isolated using EasySep Human Naive CD4+ T cell Enrichment Kit (19155 , , ).
- The resulting cell population contained 95–98% CD4+ T cells of which more than 96% were CD45RA+.
- The purified naïve CD4+ T cells were cultured in serum-free X-VIVO 15 medium (1041, , ) at 37°C, 5% CO2 at a cell concentration of 1×106 cells/ml in flat-bottomed 24-well tissue culture plates (142475) from Nunc, and stimulated with Dynabeads Human T-Activator CD3/CD28 beads (111.31D , , ) at a cell to bead ratio of 5∶1 for 3 days.
- Cells present in the culture…
Human MC culture
- Human peripheral blood derived CD34+ cells (StemCell Technologies, Vancouver, Canada) were cultured in StemPro-34 SFM supplemented with 2 mM L-glutamine, 50 µg/ml streptomycin, 100 U/ml penicillin, 100 ng/ml SCF, and 100 ng/ml recombinant human IL-6 .
- Recombinant human IL-3 (30 ng/ml) was added for the first week.
- Half of the culture medium was replaced every 7 days.
- Cultures at 8 to 10 weeks consisted of greater than 99% huMC
Wnt Signaling Experiments
- For Wnt signaling experiments, mono- and co-culture systems were used.
- In monoculture, 8×104 CD34+-ECs were seeded on the Matrigel-coated transwell insert.
- The cells were then incubated with agonists/ligands (6.25 ng/mL–100 ng/mL Wnt3A , 6.25 ng/mL–250 ng/mL Wnt7A or 0.5–5 µM for 1 or 5 days.
- Co-cultures were prepared as described before.
- The CD34+-derived ECs co-cultured with pericytes for 1 or 6 days were used in the signaling experiments.
- Agonist (0.5–5 µM BIO) was added into the basolateral compartment while antagonist (0.1–3 µM XAV939 was added in the apical part of the transwell system.
Cell Culture
- CD34+ bone marrow cells were received fresh and were plated and expanded immediately upon receipt.
- Cells were expanded for 3–5 days in StemSpan SFEM before being passaged for media formulation experiments.
- StemSpan SFEM media was supplemented with 100 ng/mL SCF, 100 ng/mL FLT3, 25 ng/mL IL6, and 25 ng/mL IL3 during expansion.
- Human embryonic stem cell line SA181 (Cellartis AB, Goteborg, Sweden) was cultured on matrigel coated flasks in medium'>TesR2 medium during expansion.
- ESCs were split for reprogramming experiments using TrypLE .
- Human adult female cryopreserved hepatocytes ( .
- F00995, Celsis/In Vitro Technologies, Chicago, IL) were thawed in medium'>CHRM medium and plated in Williams medium E supplemented with Primary Hepatocyte Thawing and Plating Supplement Pack .
- BJ fibroblasts and MRC-5 fibroblasts were thawed and plated directly into DMEM/F12+Glutamax with…
Cell culture
- Human peripheral blood-derived primary cultured MC (hPBDMC) and cord blood-derived primary cultured MC (hCBDMC) were developed from CD34+ progenitors as previously described with minor modifications + progenitors were isolated using the EasySep human CD34 positive selection kit .
- CD34+ cells from peripheral blood were cultured at 5×104 cells/mL in StemSpan SFEM supplemented with 100 ng/mL rhSCF and 100 ng/mL rhIL-6 for 8 wk, with 30 ng/mL rhIL-3 used for the first wk only for hPBDMC cultures.
- CD34+ cells from cord blood were cultured in AIM-V supplemented with 100 ng/mL rhSCF for 8 wk to develop hCBDMC.
- The StemSpan SFEM or AIM-V was hemidepleted twice a wk.
- At 4 wk, the entire volume of old media was replaced once by fresh media and then hemidepleted twice a wk until 8 wk.
- Primary MC cultures were used after 8 wk and confirmed as> 99% MC by tryptase/chymase…
-
CD34 Cord blood (CB) samples were obtained from the Hannover Medical School following written consent of the donors as approved by the Hannover Medical School local ethics committee. - Total nucleated cells were isolated by a Ficoll gradient followed by enrichment for CD34+ cells employing MACS purification .
- Isolated cells were frozen until further usage.
- CB-CD34+ cells were cultured in medium'>StemSpan medium supplemented with 1% penicillin/streptomycin and one of four cytokine combinations using hSCF, hTHPO, hFLT3-L, hGCSF, hIGFBP2, hAngptl5, hFGF-1, and hIL6 .
- The StemRegenin compound was used at a concentration of 1 µmol/l as described.