Human Interleukin-6 Recombinant (E.coli)
Categories: HematopoietinsIL-6 gp130 familyRecombinant Human Cytokines$70.00 – $2,700.00
Description
Accession
P05231
Source
Optimized DNA sequence encoding Human Interleukin-6 mature chain was expressed in E.Coli
Molecular weight
Native human Interleukin-6 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 21 kDa. Recombinant IL-6 is a homodimer protein consisting of 184 amino acid residue subunits, and migrates as an approximately 21 kDa protein under reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation ofmurineTD1 cells was found to be less than 0.1 ng/ml, corresponding to specific activity of 1x107 IU/mg.
Protein Sequence
MNSFSTSAFG PVAFSLGLLL VLPAAFPAPV PPGEDSKDVA APHRQPLTSS ERIDKQIRYI LDGISALRKE TCNKSNMCES SKEALAENNL NLPKMAEKDG CFQSGFNEET CLVKIITGLL EFEVYLEYLQ NRFESSEEQA RAVQMSTKVL IQFLQKKAKN LDAITTPDPT TNASLLTKLQ AQNQWLQDMT THLILRSFKE FLQSSLRALR QM
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Interleukin-6 was lyophilized from a 0.2 μm filtered PBS solution pH7.0.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Interactor
Interactor
Interactor
Biological Process
Molecular function
Molecular function
Methods
Gene Delivery into CD34+ Cells by Retrovirus
- The CD34+ cells isolated from cord blood were cultured in X-VIVO15 , supplemented with 1% human serum albumin (HSA) and stimulated with a cytokine cocktail [100 ng/ml stem cell factor, 100 ng/ml Flt-3 ligand, 100 ng/ml thrombopoietin, and 100 ng/ml IL-6 ] in a 24-well plate (2×105 per well) for 48 h. The stimulated CD34+ cells were then harvested and placed into non-tissue culture-treated 6-well plates that had been coated with 20 µg/ml CH-296, a recombinant fibronectin fragment .
- (3×105 cells per well) in the presence of the respective virus supernatant.
- The virus supernatants were diluted 1∶2 with X-VIVO15 containing 1% HSA and the cytokine cocktail described above.
- Every 12 h, the medium was replaced with fresh virus supernatant.
- After 48 h of culture, the frequency of GFP- and/or Venus-expressing CD34+ cells was examined by FACS.
Clonogenic assay of primary tumor cells
- Mononuclear cells (MNCs) isolated from bone marrow samples using density gradient centrifugation were treated with IFN-γ and BCGV for 2 days.
- Then CD138+ and CD138− fractions were isolated from treated-MNCs using CD138 microbeads and an AutoMACS magnetic cell sorter .
- The CD138− fraction was further depleted of normal hematopoietic progenitors using CD34, CD3, CD4 and CD8 microbeads .
- The resulting two fractions (CD138+CD34−CD3−CD4−CD8− and CD138− CD34−CD3−CD4−CD8− cells; 0.5–2.5 × 105/ml) were plated with or without GV-Th2 cells at a ratio of 1:2–5 in a methylcellulose culture system , as described above for U266 cells, containing rhIL-6 (10 ng/ml).
- Tumor colonies were counted after 2–3 weeks of culture.
- The phenotype of the cells in these colonies was confirmed by flow cytometry.
Neural in vitro differentiation
-
Neural differentiation of LLC6P and LLC9P cells was performed as previously described
g for 5 minutes at 4°C and plated on polyornithine/laminin-coated cell culture dishes. - Passage number of LLC6P hpESCs at differentiation induction was 30–45; line LLC9P cells were used at passage numbers 48–60.
- Terminal differentiation of hpNSCs was performed in differentiation media containing DMEM/F12 (N2 supplement; 1∶50) and Neurobasal (B27 supplement; 1∶50) mixed at 1∶1 ratio.
- cAMP (300 ng/mL) was added to the media for 28 days.
- For induction of dopaminergic differentiation
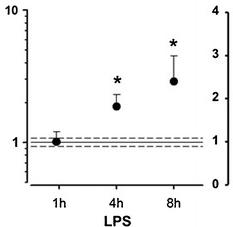
CysLT1 receptor expression in human coronary artery smooth muscle cells is upregulated by pro-inflammatory stimuli.
- Real-time quantitative TaqMan RT-PCR for CysLT receptor mRNA in SMCs incubated in the absence and presence of LPS (10 μg/mL) for 1, 4, and 8 h (a) and IL-6 (20 ng/mL), TNF-α (10 ng/mL), or IFN-γ (20 ng/mL) for either 8 h (b) or 24 h (c).
DC Co-Cultures
- Sorted naïve (live, singlet, CD3+CD28+CD95-CCR7+CD27+) CD4+ T cells were co-cultured with sorted CD103+ DCs (live, lineage-CD14-HLA-DR+CD11c+) (100:1), sorted CD103− DCs (lineage-CD14-HLA-DR+CD11c+CD103-) (100:1), or anti-CD3/anti-CD28 beads (control, 4:1) in X-Vivo15 media under Th17 conditions (10 U/mL recombinant human IL-2, 12.5 ng/mL rhIL-1β, 25 ng/mL rhIL-21, 25 ng/ml rhIL-23, 10 μg/mL anti-IFNγ, 10 μg/mL anti-IL-12 and 2 ng/mL TGF-β, with or without 25 ng/mL rhIL-6).
- All cytokines were from , except IL-2, IL-6 and IL-21 .
- DC co-cultures were stimulated with SEB (1 μg/mL ).
- All cultures were fed on day 3 with rhIL-2 (2 U/mL), anti-IFNγ (10 μg/mL) and anti-IL-12 (10 μg/mL).
- After 7 days of co-culture, qRT-PCR was performed for
IL17A andRORc gene expression . - Gene expression was quantified using ΔΔCT analysis in excel (version 12.3.0).
Culture of human mast cells
- Human umbilical cord blood was collected from mothers who had normal uncomplicated deliveries at Tufts Medical Center.
- Human cord blood-derived cultured mast cells (hCBMCs) were prepared using hematopoetic stem cells (C34+) isolated by positive selection of C34+/AC133+ cells by magnetic cell sorting using an AC133+ cell isolation kit as previously reported [+ cells were grown in medium'>medium'>medium'>serum-free expansion medium'>medium , supplemented with 100 ng/ml recombinant human stem cell factor (rhSCF; kindly supplied by Orphan , , ), 100 U/ml penicillin, 100 μg/ml streptomycin and IL-3 for the first 3 weeks, then in the medium'>medium'>medium'>serum-free expansion medium'>medium with 50 ng/ml IL-6 (ocky , , ) and for 8 to 10 weeks, with fetal bovine serum (/Gibco, , , ) added from week 6.
- The purity of the hCBMCs was…
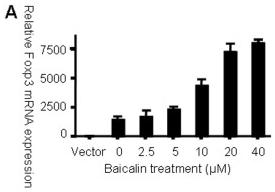
Baicalin up-regulates Foxp3 expression in HEK 293 T cells.
- HEK 293 T cells were treated with IL-6 in the presence or absence of Baicalin for 24 h, and IL-6R mRNA levels were examined by RT-PCR.
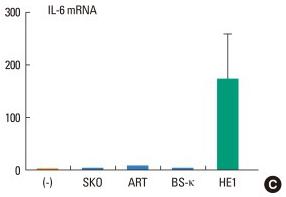
A human IgE preparation can induce IL-8 and IL-6 production and promote survival in human mast cells.
- IL-8, IL-6, and GAPDH mRNA levels were measured by quantitative RT-PCR.
Human Mast Cell Culture
- Human cord blood-derived cultured mast cells (hCBMCs) were cultured as described below.
- Human umbilical cord blood was collected following normal uncomplicated deliveries at Tufts Medical Center (Boston, MA) under Exemption #4 (discarded blood without identifiers) approved by the Tufts Medical Center Human Institutional Review Board (IRB).
- No consent form was required for discarded tissue.
- Cord blood cells were isolated by positive selection of C34+/AC133+ cells with magnetic cell sorting using an AC133+ cell isolation kit as previously reported + cells were suspended in StemSpan serum-free expansion medium , supplemented with 100 ng/ml rhSCF; kindly supplied by Sweden Orphan and 100 U/ml penicillin/100 µg/ml streptomycin , and IL-3 for the first 3 weeks, and 50 ng/ml IL-6 (ocky , ) for 8 to 10 weeks.
- Fetal bovine serum (FBS, , , ) was added from week 6 on.
- The purity of hCBMCs was evaluated…
Inflammatory conditions reduce Imatinib uptake and hMATE1 expression in hRASF.
- B, C, and D) Effect of 18 hours incubation with a TNFα, IL-1β and IL-6 (+sIL-6R) cocktail and with single cytokines (each at 10 ng/ml) on Imatinib uptake in hRASF , hMATE1-mRNA expression , hMATE1-protein expression by immunofluorescence staining (upper part of D) and by Western Blot analysis of biotynilated plasma membrane fractions hMATE1 (lower part of D showing an example of a typical Western blot together with the quantitative analysis of three independent experiments).