Human Interleukin-6 Recombinant (E.coli)
Categories: HematopoietinsIL-6 gp130 familyRecombinant Human Cytokines$70.00 – $2,700.00
Description
Accession
P05231
Source
Optimized DNA sequence encoding Human Interleukin-6 mature chain was expressed in E.Coli
Molecular weight
Native human Interleukin-6 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 21 kDa. Recombinant IL-6 is a homodimer protein consisting of 184 amino acid residue subunits, and migrates as an approximately 21 kDa protein under reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation ofmurineTD1 cells was found to be less than 0.1 ng/ml, corresponding to specific activity of 1x107 IU/mg.
Protein Sequence
MNSFSTSAFG PVAFSLGLLL VLPAAFPAPV PPGEDSKDVA APHRQPLTSS ERIDKQIRYI LDGISALRKE TCNKSNMCES SKEALAENNL NLPKMAEKDG CFQSGFNEET CLVKIITGLL EFEVYLEYLQ NRFESSEEQA RAVQMSTKVL IQFLQKKAKN LDAITTPDPT TNASLLTKLQ AQNQWLQDMT THLILRSFKE FLQSSLRALR QM
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Interleukin-6 was lyophilized from a 0.2 μm filtered PBS solution pH7.0.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Interactor
Interactor
Interactor
Biological Process
Molecular function
Molecular function
Methods
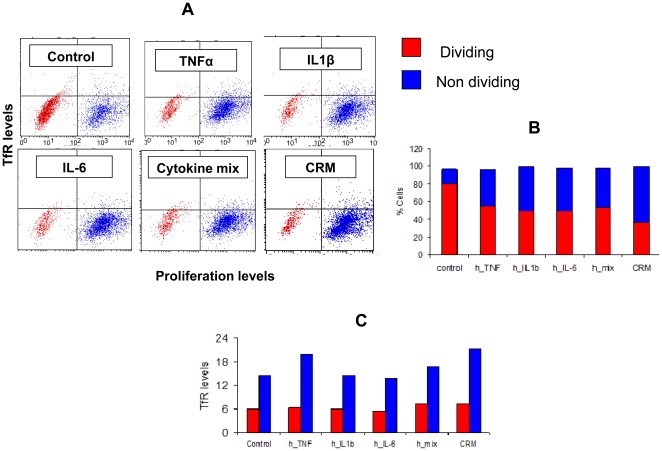
Increased TfR expression in CaCo-2 cells is not due to enhanced proliferation.
- Flow cytometry analysis of CaCo-2 cells double stained for CFSE dilution assay and TfR (mouse anti-human TfR stain), after incubation with the following inflammation mediators: TNFα, IL1β, IL-6, a mixture of the three, or CRM from mouse source.
Cells and cultures
- The human KG1 leukemic cell line, RPMI8226 myeloma (MM) cell line, HepG2 hepatoma cell line, DU145 prostate carcinoma cell line, and A-MB231 breast cancer cell lines were obtained from American Type Culture Collection (ATCC) .
- The MM cell line INA6 was kindly provided by Dr. Renate Burger (University of Kiel, Kiel, Germany).
- Bone marrow mononuclear cells (BMMCs) were isolated from fresh bone marrow aspirates of patients with myeloma and primary CD138+ myeloma cells were further sorted using CD138 MicroBeads as described previously
Processing whole blood samples
- Peripheral (PB.1 and PB.2) and cord (CB.1 and CB.2) blood-derived CD34+ cells were obtained from AllCells .
- Blood collections were performed at AllCells and using standard, 8 ml Vacutainer Cell Processing Tubes (both sodium citrate and sodium heparin-based tubes are acceptable ; , ).
- Appropriate documentation for informed consent was completed prior to blood collection .
- Vacutainers were processed within 24 hours of collection.
- Briefly, the PBMC-containing upper phase was collected and washed with ice-cold PBS .
- Cells were either frozen down or used directly for purification with the CD34 MicroBead Kit and used according to the manufacturer's protocol.
- Some samples were treated with Histopaque ( ; St. Louis, ) to minimize the number of red blood cells and centrifuged at 2000 rpm for 20 minutes without braking.
- The interface containing the PBMCs was removed if samples were treated with histopaque, cells washed again with chilled…
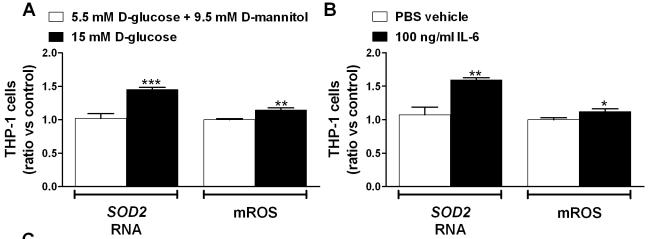
Exposure of IRAK3-depleted THP-1 cells to additional stress results in more inflammation and ROS.
- Gene expression was analyzed using qRT-PCR and mROS production was determined by flow cytometry in THP-1 cells exposed to 5.5 mM D-glucose and 9.5 mM D-mannitol (osmotic control) or 15 mM D-glucose (n = 6), and 100 ng/ml IL-6 (n = 6).
Isolation of PBMCs and Monocyte-derived DC Generation
- The DC generation procedure was followed as previously described by Romani et al.
- and Kim et al.
- 6/well) were incubated for 5 days in medium'>AIM medium'>V medium containing 800 U/mL of animal-free (AF) recombinant interleukin 4 (rhIL-4) and 1000 U/mL AF-recombinant granulocyte-monocyte colony-stimulating factor (rhGM-CSF) to induce immature DC (iDC).
- Fresh medium containing these cytokines was replaced every 3 days.
- On day 5, to induce mature DCs (mDCs), iDCs were exposed to a cytokine cocktail containing AF-recombinant IL-1β (1000 U/mL) , IL-6 (1000 U/mL) and TNF-α (1000 U/mL) .
- After 6 h, a 10 µg/mL final concentration of PolyI:C was added to iDCs and cultured for 48 h. At day 7 or 8, the DC culture supernatant was collected and frozen at −80°C.
- Then, mDCs were harvested and stained with PE or FITC-conjugated antibodies…
Cell Culture
- Frozen female CD34+ CBCs were supplied by Bio-Resource Center .
- C34+ CBCs were cultured in hematopoietic culture medium [serum-free X-Vivo10 containing 50 ng/mL IL-6 , 50 ng/mL sIL-6 , 50 ng/mL SCF , ten ng/mL TPO , and 20 ng/mL Flt3/4 ligand ].
- Reprogrammed cells were cultured in feeder-less primate ES cell medium Repro FF (, .
- No. RCHEMD004), ReproFF2 (ReproCELL, cat No. RCHEMD006), mTeSR1 ( catalog number 05850) or E8 (16) supplemented with five ng/mL bFGF (total bFGF ten ng/mL) on Pronectin F-coated dishes.
- Passage of human iPSCs was previously described
Cell cultures
- Cord blood-derived mast cells were derived in supplemented medium'>StemPro-34 SFM medium including 100 ng/mL recombinant human SCF (hSCF , , ) and 10 ng/mL human IL-6 as previously described
Generation of DC
- Blood samples from healthy donors are collected after informed consent, in accordance with the Declaration of Helsinki and approval of the of the University Hospital of the Ludwig-Maximilians-University, Munich, Germany.
- Peripheral blood mononuclear cells (PBMC) are isolated by Ficoll density gradient centrifugation.
- PBMC are resuspended in 15 ml VLE (very low endotoxin) medium'>RPMI 1640 medium supplemented with 1.5% human serum medium'>(DC medium) at 7.5′107 cells per 75 cm2 culture flask (NUNC, 178905) and incubated at 37°C and 5% CO2 for 1 h. Non-adherent cells are carefully removed by washing.
- Adherent monocytes are cultured in medium containing 100 ng/ml GM-CSF (Leukine® by Berlex, NC50419-050-30) and 20 ng/ml interleukin-4 (& 104-IL-050-CF) and fed with the same medium on days 3 and 6.
- On day 6 of culture, the immature C are differentiated into mC by addition of medium containing 10 ng/ml IL-1β…
Purification and Transduction of Mouse and Human Hematopoietic Progenitors
- Mouse Hematopoietic Progenitors (mLin−) were purified from BM mononuclear cells of C57BL6/6J mice byCell Depletion .
- The mouse cells were then prestimulated in StemSpam SFEM (serum-free expansion medium, StemCells Technologies, Canada) with 50ng/mL mouse Stem Cell Factor (mSCF) , 100ng/mL human Interleukin-11 (hIL-11), 100ng/mL human Flt-3 Ligand (hFlt-3L) and 10ng/mL human Interleukin-3 (hIL-3) (ocky , , ) for 4-6 hours.
- After which, mLin− cells were transduced with the different lentivirus.
- On the other hand, cord blood was collected from mothers attending the Royal Hospital, , after informed consent and via a protocol approved by the Local Research Ethics.
- Mononuclear cells (MNC) were obtained by Ficoll density centrifugation and ammonium chloride red cell lysis.
- Density-separated CB MNCs were depleted for lineage marker positive cells via theSep™ system ( Cell , ) according to the manufacturer’s instructions to generate Lineage negative (Lin−)…
Liquid Cultures assays
- Two types of liquid culture were used one for maintaining stem/progenitors and the second for inducing myeloid differentiation.
- For the maintenance of HSC/progenitors, transduced Lin− cells were cultured in IMDM / 10% Fetal Calf Serum (FCS) supplemented with 20ng/mL SCF, 50ng/mL Interleukin-3 (IL-3), 20ng/mL IL-6 and 10ng/mL Granulocyte-Colony Stimulating Factor (G-CSF) .
- Fresh media was added every week.
- For Myeloid promoting differentiation, transduced cells were cultured as previously described for two weeks ( in IMDM/15% FCS supplemented with 2ng/ml IL-3 and 20ng/ml SCF.