Human Interleukin-18 Recombinant
Categories: Interleukin-1 familyRecombinant Human Cytokines$70.00 – $3,600.00
Description
Accession
Q14116
Source
Optimized DNA sequence encoding Human Interleukin-18 mature chain was expressed in Escherichia Coli
Molecular weight
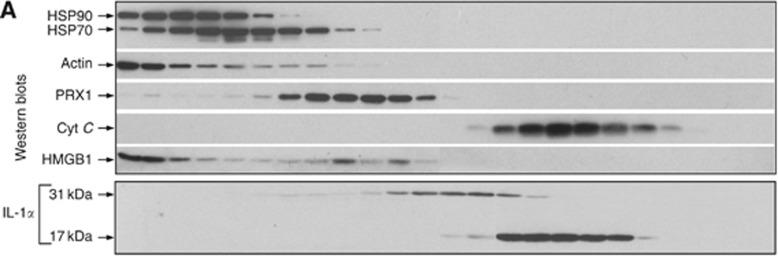
Native human Interleukin-18, generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calculated molecular mass of approximately 18kDa. Recombinant Interleukin-18 is a monomer protein consisting of 157 amino acid residue subunits, migrates as an approximately 18 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>92%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent proliferation ofhuman PBMC co-stimulated with IL-12 cells was found to be in the range of 5ng/ml.
Protein Sequence
YFGKLESKLSVIRNLNDQVLFIDQGNRPLFEDMTDSDCRDNAPRTIFIISMYKDSQPRGMAVTISVKCEKISTLSCENKIISFKEMNPPDNIKDTKSDIIFFQRSVPGHDNKMQFESSSYEGYFLACEKERDLFKLILKKEDELGDRSIMFTVQNED
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Interleukin-18 was lyophilized from.2 μm filtered PBS solution pH7.4 .
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Q9IW12
Interactor
Q99665
Molecular function
Methods
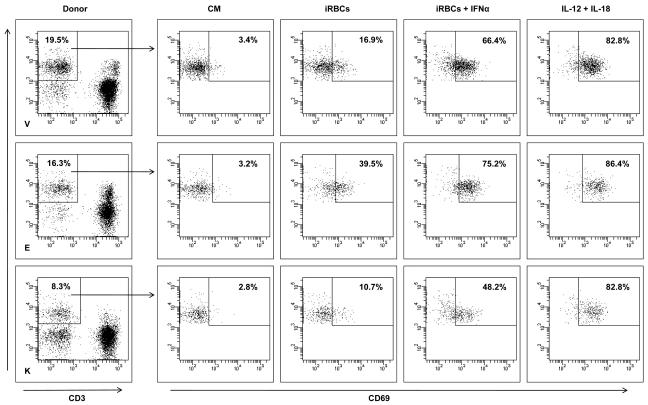
Regulation of CD69 and IFN-γ on NK cells.
- PBMCs from the three donors were incubated with 3D7 schizont-infected erythrocytes (iRBCs), with iRBCs plus human IFN-α or with a mixture of IL-12 and IL-18, or were leftuntreated in culture medium for 24 hours and analyzed by flow cytometry.
NK92/iRBCs co-culture and flow cytometry
- NK92 cells were kept in two different environments for 24 hours prior to the co-culture: in normal cell medium'>medium medium'>(+rIL2; NK92 nm) and in cell medium'>medium without rIL-2 (starvation medium'>medium; NK92s).
- Cells from both environments were co-cultured with 3D7 schizont-infected erythrocytes and uRBCs (NK92-RBCs ratio: 1:3) in their respective growth medium.
- As a positive control, cells were also incubated with a mixture of IL-12 and IL-18 (and MBL, respectively; 100 ng/106 cells each).
- After the indicated time of incubation at 37°C and 5% CO2, NK cells from the co-culture as well as cells incubated without RBCs were stained for 30 min at 4°C with fluorochrome-conjugated antibodies for surface CD56 (APC), CD3 (PE), CD16 (FITC), CD69 (FITC), CD25 (PE) in parallel with the appropriate isotype controls.
- Cells were also internally stained with the IFN-γ (PE) antibody (all…
Influence of selected cytokines and growth factors on the IL-33 synthesis in RA-SFs.
- RA-SFs (n=4) were stimulated for 24 h with TNF-α, IL-1β, IL-18, PDGF-BB or TGF-1β (concentrations as indicated in the figure).
Influence of selected cytokines and growth factors on the IL-33 synthesis in RA-SFs.
- RA-SFs (n=4) were stimulated for 24 h with TNF-α, IL-1β, IL-18, PDGF-BB or TGF-1β (concentrations as indicated in the figure).
Cell isolation and stimulation
- For flow cytometry analysis and cell sorting of adult blood, fluorescently-labeled antibodies to the following antigens were used, CD3 (UCHT1), CD16 (3G8) CD56 (HCD56) and CD94 (DX22, , ); for cord blood, CD34 (581), CD117 (104D2), CD94 (HP-3D9), CD3 (UCHT1), and CD56 (NM16.2) .
- medium'>NK cells (1 × 106) were incubated in 1 ml of medium'>NK complete medium for 24 h at 37°C in 5% CO2 with IL-2 (100 U/ml, Biologicalesources Branch, National Cancer Institute, Frederick, M), IL-12 (10 U/ml, , ) plus IL-18 (100 ng/ml& , , ), IL-15 (100 ng/ml), TGF-β (10 ng/ml& ), or PMA (10 ng/ml, ) plus the calcium ionophore, A23187 (250 ng/ml, , ).