Human Interleukin-15 Recombinant
Categories: HematopoietinsIL-2 familyRecombinant Human Cytokines$70.00 – $3,500.00
Description
Accession
P40933
Source
Optimized DNA sequence encoding Human Interleukin-15 mature chain was expressed in Escherichia Coli.
Molecular weight
Native human Interleukin-15 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 13 kDa. Recombinant Interleukin-15 is a monomer protein consisting of 115 amino acid residue subunits, and migrates as an approximately 13kDa protein under reducing conditions in SDS-PAGE.
Purity
>96%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent proliferation ofmouseCTLL-2 cells and was found less than0.3 ng/ml.
Protein Sequence
MRISKPHLRS ISIQCYLCLL LNSHFLTEAG IHVFILGCFS AGLPKTEANW VNVISDLKKI EDLIQSMHID ATLYTESDVH PSCKVTAMKC FLLELQVISL ESGDASIHDT VENLIILANN SLSSNGNVTE SGCKECEELE EKNIKEFLQS FVHIVQMFIN TS
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than.1 ng/µg(1EU/µg).
Presentation
Recombinant IL-15 was lyophilized from a.2 μm filtered PBS solution pH7.5 .
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at° -° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Interactor
Molecular function
Methods
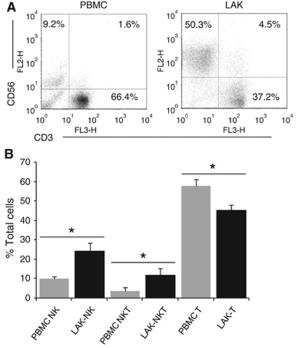
Isolation of MiHA specific T-cells by pMHC tetramer pull down
- Prior to isolation of peptide-specific T-cells, pMHC tetramers were made coupled to SA-PE.
- PBMC were stained with pMHC tetramers for 1 hour at 4°C.
- Subsequently, cells were washed and incubated with anti-PE Ab coated magnetic beads .
- Cells were than isolated by MACS , using an LS column, following the manufacturers protocol.
- Eluted cells were washed and cultured in Iscove Modified Dulbecco Medium (IMDM; ) supplemented with 5% human serum, 5% fetal calf serum (FCS), 100 IU/mL IL-2 , 10ng/mL IL-15 .
- Eluted cells were cultured per 5000 cells with 2×104 irradiated autologous feeder cells and 5000 anti-CD3/CD28 Dynabeads in 96-well plates.
- Cultures were split at least twice a week.
- After 2–3 weeks, cell cultures were analyzed for peptide-specific T-cell populations by pMHC tetramer flow cytometry.
- Subsequently pMHC tetramer reactive T-cell populations were sorted on a FACSAria into 96 well plates containing 1×105 irradiated feeder cells supplemented…
Cell isolation and culture
- Peripheral blood mononuclear cells (PBMC) from CLL patients or healthy donors were separated from heparinized venous blood by Ficoll density gradient centrifugation.
- The PBMC used in this study were generally greater than 90% CLL B cells as determined by CD19/CD5 positivity.
- Leukemic cells were cultured in serum-free adoptive immunotherapy media-V medium'>(AIM-V) medium .
- For CLL B cell activation experiments, cells were cultured for 5 days in AIM-V media or AIM-V media supplemented with 2.5 µg/ml CpG ODN 2006 (provided by core Mayo Clinic facility) with or without 94 IU/ml human IL-2 , 10 ng/ml human IL-15 , and 13 µM beta-mercaptoethanol.
- Further CLL B cell activation experiments utilized 12-O-tetradecanoylphorbol-13-acetate (TPA) at 10 ng/ml or interferon-α 2b at 1000 IU/ml.
- Human C4+T cells were isolated from healthy donor PBMC by immunomagnetic selection using T cell enrichment kits and incubated with CLL B cells…
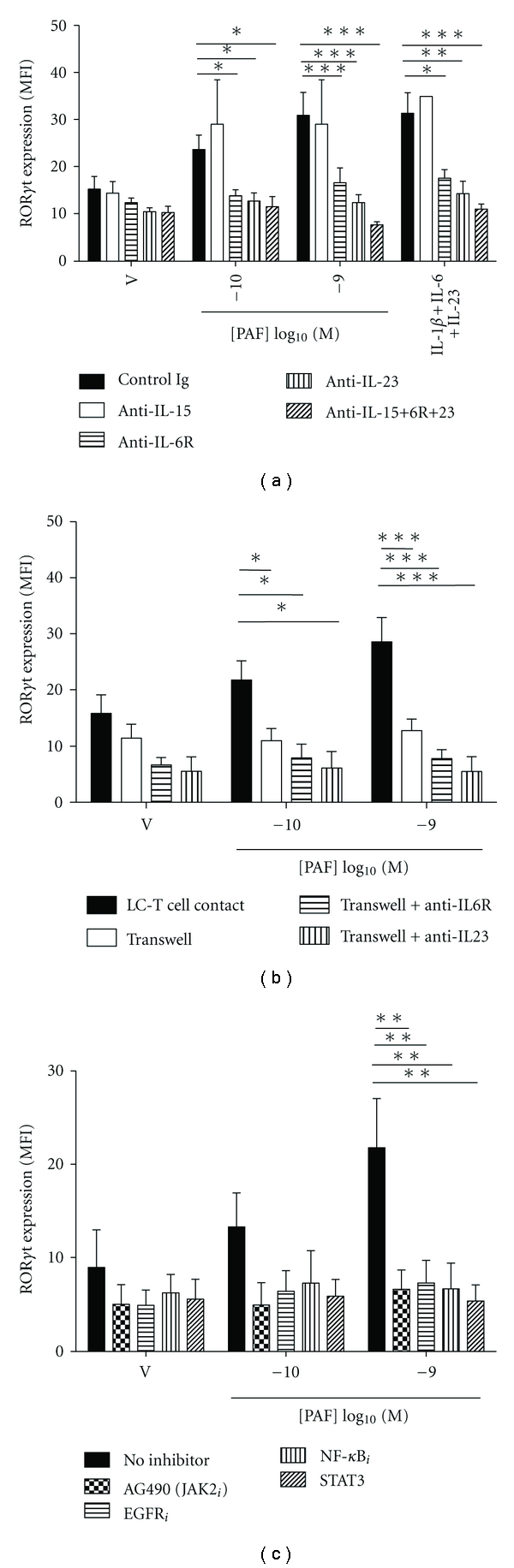
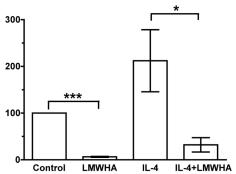
PAF-stimulated Th17 development is dependent on cytokines, LC-T cell contact and selected signaling pathways.
- (a) Monocyte-derived LC were stimulated with either vehicle or PAF in the presence of neutralizing Ab to IL-15, IL-6R, and/or IL-23, or control Ig, and cocultured with antiCD3/CD28-activated CD4+ T cells for 5 days.
Donor APCs plus γc cytokines induce rapid expansion of CD8+CD28− T cells in culture.
- Freshly purified CD8+ T cells (2×106 per well) from healthy volunteers were stimulated by HLA-A, B and DR mismatched allogeneic APCs (1×106 per well) for 9 days in 24-well plates supplemented with IL-2 alone or a combination of IL-2, IL-7 and IL-15.
Human in vitro iNKT cell assay
- Human iNKT cell clones have been previously described.
- Human primary iNKT cell lines were generated by expanding freshly isolated PBMC in 50 U/ml IL-2 and 5 ng/ml IL-15 in culture with 10 ng/ml α-GalCer for 14 days.
- iNKT cells were purified by PBS-57-loaded CD1d tetramer selection with magnetic beads , and were > 99% CD3+PBS-57-tet+.
- Generation of human PBMC-derived monocytes has been previously described.
- 5 × 104 iNKT cells were cultured with 5 × 104 PBMC-derived monocytes per well.
The co-culture model system
- The in vitro model simulating the human tumor microenvironment contained 5 × 105 iDC co-cultured with 5 × 105 irradiated (3,000 rad) PCI-13 cells and autologous CD4+CD25− T cells (5 × 106) in six-well plates [2 in air at 37°C for 10 days.
- In addition, 2.5 mL aliquots of IRX-2 or X-Vivo 10 medium (control) were added to each well as well as rhIL-2 (10 IU/mL), IL-10 (20 IU/mL), and IL-15 (20 IU/mL) .
- On days 3, 6, and 9, half of the medium was removed and replaced with fresh cytokine-containing medium mixed 1:1 with medium or IRX-2.
- For cytokine assays, cells were stimulated for 16 h with anti-CD3/CD28-coated beads at the bead:cell ratio of 1:1 in complete medium'>AIM V medium without exogenous cytokines or IRX-2.
- For intracellular cytokine staining, Brefeldin A (2 μg/mL, , ) was added to the cells.
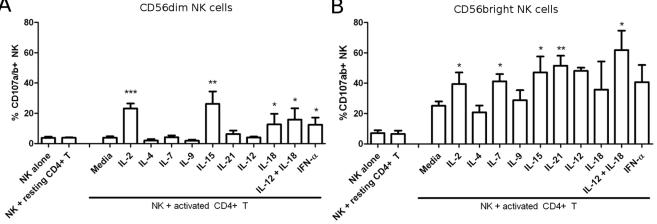
CD56bright NK cells have a higher cytotoxic potential towards activated CD4+ T cells.
- NK cells were activated as indicated: 200 IU/mL IL-2, 25 ng/mL IL-4, 25 ng/mL IL-7, 25 ng/mL IL-9, 5 ug/mL IL-15, 100 ug/mL IL-21, 50 ug/mL IL-12, 0.25 mg/mL IL-18 or 100 U/mL IFN-αA.
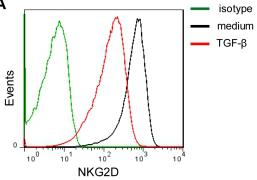
Anti-TGF-β1 partially restores the surface expression of NKG2D and 2B4 on NK cells.
- Histograms correspond to NK cells from one representative donor treated with the isotype controls (green lines), medium alone (RPMI 1640 supplemented with 10% FBS in the presence of IL-15 (10 ng/ml) and IL-2 (100 U/ml)) (black lines) and TGF-β1 (1 ng/ml) (red lines).
Cells
- Peripheral blood lymphocytes were isolated from healthy female donors using Ficoll gradient.
- celltype'>celltype'>NK celltype'>cells were further purified by using the human celltype'>celltype'>NK cell isolation kit and the autoMACS instrument , according to the manufacturer's instructions.
- The identity of NK cells was confirmed by FACS.
- Purity was >98%.
- LILRB1-positive NK clones were grown in culture in the presence of 10 ng/ml IL-15 and then used for NK cytotoxicity assays.