Human Interleukin-11 Recombinant
Categories: HematopoietinsIL-6 gp130 familyRecombinant Human Cytokines$70.00 – $4,700.00
Description
Accession
P20809
Source
Optimized DNA sequence encoding Human Interleukin-11 mature chain was expressed in Escherichia Coli.
Molecular weight
Native human IL-11, generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 19 kDa. Recombinant IL-11 is a monomer protein consisting of 179 amino acid residue subunits, and migrates as an approximately 19 kDa protein under nreducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of the proliferation ofmurine T11 cells is ≤.0 ng/ml, corresponding to a specific activity of ≥1 xunits/mg.
Protein Sequence
MNCVCRLVLV VLSLWPDTAV APGPPPGPPR VSPDPRAELD STVLLTRSLL ADTRQLAAQL RDKFPADGDH NLDSLPTLAM SAGALGALQL PGVLTRLRAD LLSYLRHVQW LRRAGGSSLK TLEPELGTLQ ARLDRLLRRL QLLMSRLALP QPPPDPPAPP LAPPSSAWGG IRAAHAILGG LHLTLDWAVR GLLLLKTRL
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Interleukin-11 was lyophilized from a 0.2 μm filtered PB solution in.5% glycine, pH.5.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Molecular function
Molecular function
Methods
Cell culture
- To elucidate the effect of exogenous IL-11 on the regulation of versican isoforms, we performed treatment assays with different concentrations of rhIL-11.
- The gastric carcinoma cells were seeded at an equal density in cell culture flasks containing growth medium as described above.
- After 24 h, triplicate samples of subconfluent cells were treated with four different concentrations (0, 20, 50, 100 and 200 ng/ml) of activated rhIL-11 protein and incubated for 24 h. The cells were harvested to prepare mNA and protein as described below.
- In the time-point assays, the cells were treated with 100 ng/ml of rhIL-11 and harvested at three different time points: hours 24, 48 and 72.
Cell lines and cell culture
- 293T ( .
- number CRL-11268) and NIH3T3 ( .
- number CRL-1658) cells were both cultured in medium'>Dulbecco's medium'>modified medium'>Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) and 1% Penicillin-Streptomycin with 5% CO2 at 37°C.
- Bone marrow cells (BM) isolated from wild type (wt) C57BL/6 mice were cultured in StemSpan Serum-free expansion medium supplemented with 1% glutamine, 1% penicillin-streptomycin, 100 ng/mL each murine stem cell factor , human Flt3-ligand and human interleukin 11 , and 10 ng/mL murine interleukin 3 .
- The KSHV-positive PEL cell line BCBL-1
Cell Culture
- Human cord blood was collected following normal pregnancies and deliveries upon informed consent of the parents, in accordance with the ethical committee of the IRCCS Policlinico San Matteo Foundation and the principles of the Declaration of Helsinki.
- CD34+ cells from human cord blood samples were separated and cultured as previously described + progenitor cells were separated using immunomagnetic beads selection and cultured, for 13 days, in medium'>Stem medium'>Span medium (medium'>Stem cell; , ) supplemented with 1% L-glutamine, 100 U/ml penicillin, 100 ng/mL streptomicyn, 10 ng/mL IL-6, IL-11, 10 or 100 ng/mL rHuTPO ( EC , , ), and 100, 1000 or 2000 ng/mL AMG531 at 37°C in a 5% CO2 fully humified atmosphere.
- celltype'>MO7e cell line (Genetics Institute, Boston, MA) was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 1% L-glutamine,…
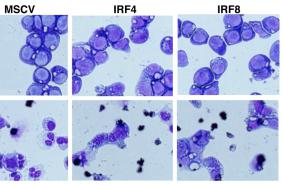
Myeloid Immortalization Assay
- We essentially followed the protocol developed by Modlich et al for the myeloid immortalization assay -) BM cells were isolated from 5 female C57BL6/J mice (6–8 weeks old ) using Lineage Cell Depletion Kit .
- Lin– BM cells were prestimulated for 2 days in’s Modified Dulbecco’s Medium containing 50 ng/ml recombinant murine stem cell factor, 100 ng/ml recombinant human Flt-3 ligand, 100 ng/ml recombinant human IL-11, 10 ng/ml recombinant murine IL-3 , 10% fetal calf serum, 1% penicillin/streptomycin, and 2 mmol/l glutamine (here after refer as I10-S3F11 medium) at a density of 5×105 cells/ml.
- The cells were transduced on days 3, 4, 5, and 6 using 105 cells and an MOI of 10–30 per transduction.
- For each vector or vector dose, three replicate transductions were performed in three different wells of a 24 well tissue culture plate and vector copy number for each…