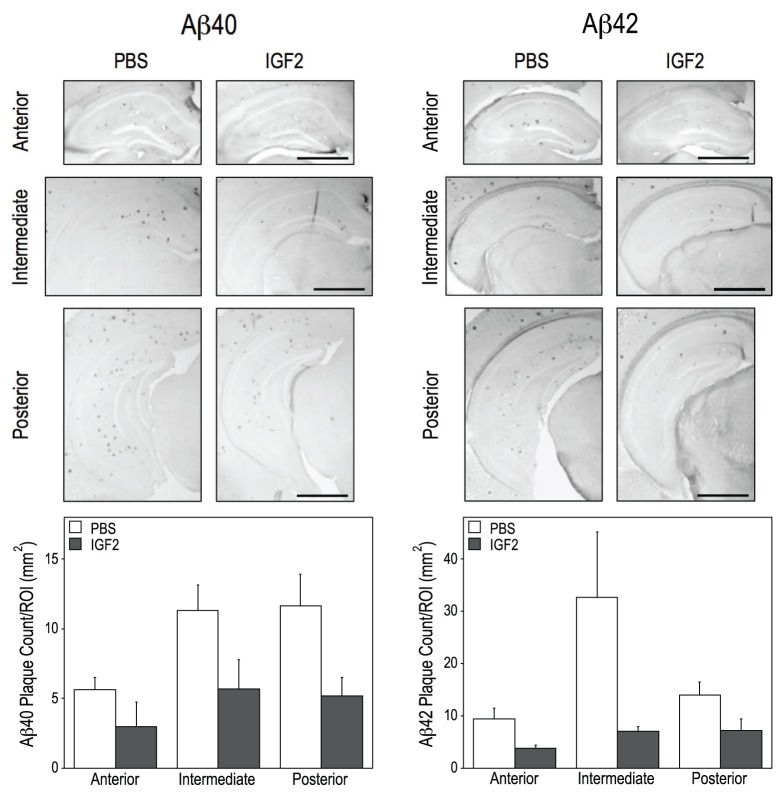
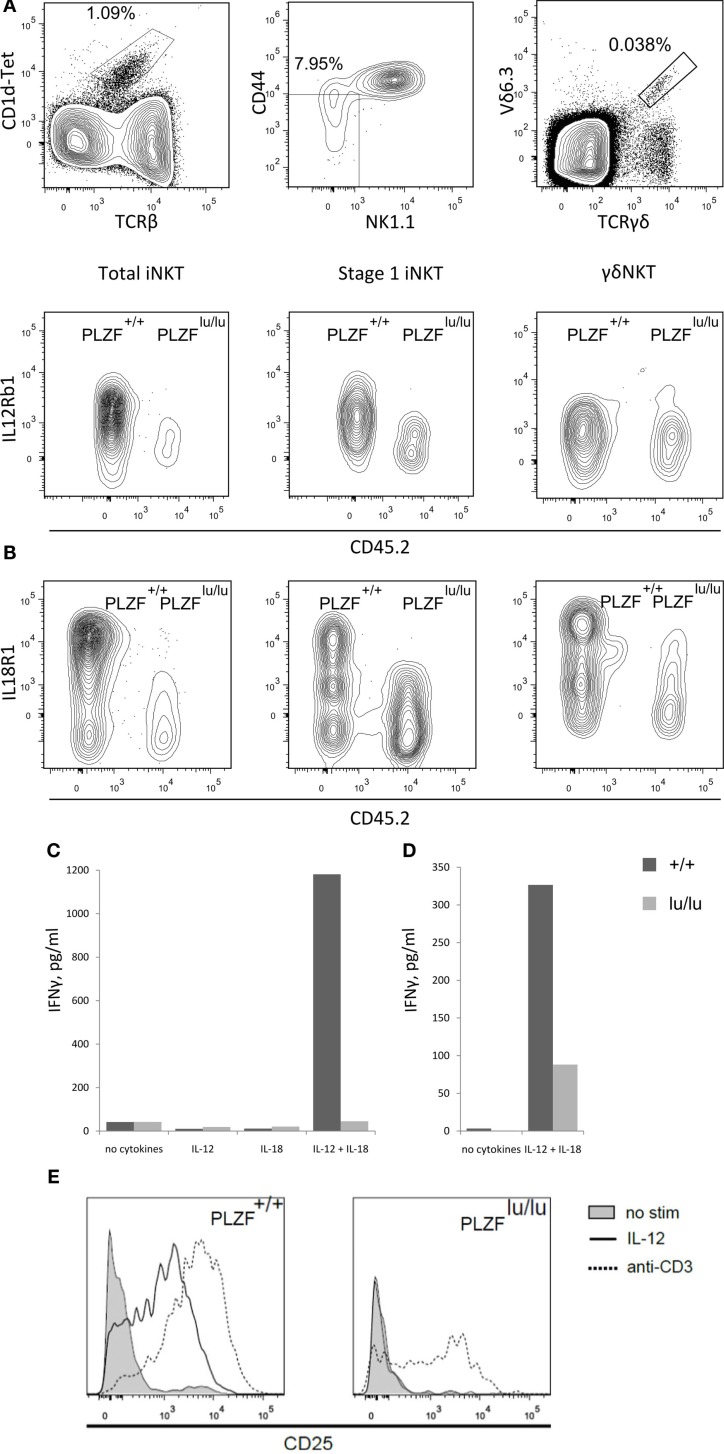
Human Interleukin 1 beta Recombinant
Categories: Interleukin-1 familyRecombinant Human Cytokines$70.00 – $3,500.00
Description
Accession
P01584
Source
Optimized DNA sequence encoding Human Interleukin-1 beta mature chain was expressed in Escherichia Coli.
Molecular weight
Mature Human IL-1 beta, is generated by the proteolytic removal of the signal peptide and propeptide.The molecule has a calculated molecular mass of approximately17 kDa. Recombinant IL-1 beta is a monomer protein consisting of 154 amino acid residue subunits. Recombinant IL-1 beta migrates as an approximately 17 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) as determined by thedose-dependent proliferation of murine D10S cells, was found to be ≤0.1 pg/ml, corresponding to a specific activity of ≥1 x107units/mg.
Protein Sequence
MAEVPELASE MMAYYSGNED DLFFEADGPK QMKCSFQDLD LCPLDGGIQL RISDHHYSKG FRQAASVVVA MDKLRKMLVP CPQTFQENDL STFFPFIFEE EPIFFDTWDN EAYVHDAPVR SLNCTLRDSQ QKSLVMSGPY ELKALHLQGQ DMEQQVVFSM SFVQGEESND KIPVALGLKE KNLYLSCVLK DDKPTLQLES VDPKNYPKKK MEKRFVFNKI EINNKLEFES AQFPNWYIST SQAENMPVFL GGTKGGQDIT DFTMQFVSS
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
IL-1 beta was lyophilized from a 0.2 μm filtered PBS pH7.0.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
P29466
Biological Process
Molecular function
Molecular function
Molecular function
Methods
Migration assays
- HUVECs were seeded at 15–20×103/well and cultured to 90% confluence on 24-well transwell tissue culture inserts (24-well plate with inserts, 5 µm pore/6.5 mm, TC-treated) as previously described 3 receptor agonists and antagonists were present during the migration in both transwell compartments.
- Migration experiments displayed an inter-donor variability in the absolute number of migrating T cells, thus for each experiment migration data have been normalized considering the number of counted cells in control conditions as 100%.
Islet isolation, culture, and exposure to different beta-cell inhibitory substances
- Following the initial culture period, islets were cultured for an additional 6–24 hours in CMRL 1066 containing antibiotics, 2 mM glutamine and one of the following supplements: 0.5 mM sodium palmitate solubilized in 0.5% (weight/volume) fatty acid and lipopolysaccaride free bovine serum albumin (BSA) ; recombinant human Interleukin 1beta (IL-1ß (50 units/ml) and Interferon-gamma (IFN-γ) (1,000 units/ml) ; 100 mM hydrogen peroxide; 2 mM DETA/NO or 10 mM streptozotocin (STZ) .
- To some of the groups 10 µM of Imatinib was added at different time points prior to the addition of test substances given above.
- To controls equal amounts of vehicle (DMSO) were supplemented.
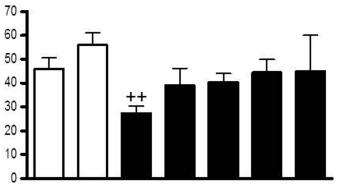
The placenta releases sST2.
- C) Placental explants release sST2, secretion was increased by treatment with TNFα and IL-1β (100 ng/ml) and hypoxia-reperfusion injury, and was decreased under hypoxic conditions (n = 3).
Effect of CORM-2 on HO-1 protein (A) and mRNA expression (B) in OA synoviocytes.
- Cells were stimulated with IL-1β (10 ng/ml) for 24 h and 16 h in the presence or absence of CORM-2 (50, 100, 200 µM) or RuCl3 (200 µM).
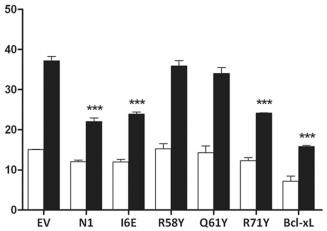
Functional characterization of mutant N1.
- Cells were treated with IL-1β, lysed and the relative fold activation of NF-κB activity was determined.
Infection of A549 cells for measurement of HBD2 gene expression and IL-8 levels
- The A549 cell line is a well characterized Type II alveolar epithelial tumor cell line which was obtained from the American Type Culture Collection .
- A549 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% Fetal Bovine Serum (FBS) and 1% Antibiotic-Antimycotic at 37°C in 5% CO2.
- All experiments were performed prior to the twenty fifth cell passage.
- A549 cells were plated at 1.5×105 cells/mL, in a volume of 3 mL of DMEM+10% FBS+1% Antibiotic-Antimycotic in 6-well tissue culture treated plates and incubated for 72 h at 37°C, in humidified 5% CO2.
- The cells were then washed 3 times with 3 mL Iscove's Modified Dulbecco's Medium (with sodium bicarbonate; without L glutamine:), and the media was replaced with 2 mL Iscoves+5% human serum ( AB Serum, ).
- Wells were infected with
M. - abscessus variants at…
2.5. DC Maturation and Rapamycin Treatment
- Two days after monocytes were plated, monocyte-derived and hESC-derived immature DC were treated with 10 ng/mL and 5–7 ng/mL of rapamycin , respectively.
- On day 5, Cs were matured for 48 hr using a maturation cocktail consisting of 50 ng/mL of GM-CSF , 100 ng/mL IL-4 , 20 ng/mL IFN
γ , 50 ng/mL TNFα , 10 ng/mL of IL-1β , and 1μ g/mL PGE2 . - On day 6-7, DCs were harvested by gentle pipetting, passed through a 70
μ m cell strainer, centrifuged, and resuspended prior to their use in experiments.
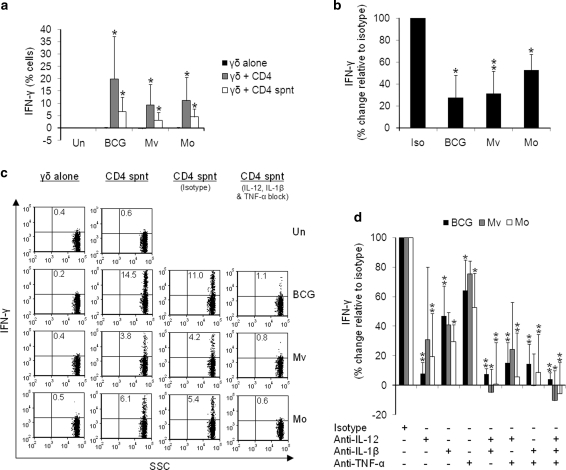
IL-12, IL-1β and TNF-α from BCG-, M. vaccae- and M. obuense-treated CD4+ cells activate Vδ2+ cells.
- b PBMCs were cultured overnight with mycobacteria in the presence of blocking antibodies to IL-12, IL-1β and TNF-α each at 100 μg/ml.
Isolation of PBMCs and Monocyte-derived DC Generation
- The DC generation procedure was followed as previously described by Romani et al.
- and Kim et al.
- 6/well) were incubated for 5 days in medium'>AIM medium'>V medium containing 800 U/mL of animal-free (AF) recombinant interleukin 4 (rhIL-4) and 1000 U/mL AF-recombinant granulocyte-monocyte colony-stimulating factor (rhGM-CSF) to induce immature DC (iDC).
- Fresh medium containing these cytokines was replaced every 3 days.
- On day 5, to induce mature DCs (mDCs), iDCs were exposed to a cytokine cocktail containing AF-recombinant IL-1β (1000 U/mL) , IL-6 (1000 U/mL) and TNF-α (1000 U/mL) .
- After 6 h, a 10 µg/mL final concentration of PolyI:C was added to iDCs and cultured for 48 h. At day 7 or 8, the DC culture supernatant was collected and frozen at −80°C.
- Then, mDCs were harvested and stained with PE or FITC-conjugated antibodies…
Generation of DC
- Blood samples from healthy donors are collected after informed consent, in accordance with the Declaration of Helsinki and approval of the of the University Hospital of the Ludwig-Maximilians-University, Munich, Germany.
- Peripheral blood mononuclear cells (PBMC) are isolated by Ficoll density gradient centrifugation.
- PBMC are resuspended in 15 ml VLE (very low endotoxin) medium'>RPMI 1640 medium supplemented with 1.5% human serum medium'>(DC medium) at 7.5′107 cells per 75 cm2 culture flask (NUNC, 178905) and incubated at 37°C and 5% CO2 for 1 h. Non-adherent cells are carefully removed by washing.
- Adherent monocytes are cultured in medium containing 100 ng/ml GM-CSF (Leukine® by Berlex, NC50419-050-30) and 20 ng/ml interleukin-4 (& 104-IL-050-CF) and fed with the same medium on days 3 and 6.
- On day 6 of culture, the immature C are differentiated into mC by addition of medium containing 10 ng/ml IL-1β…