Human Interferon-gamma Recombinant
Categories: Interferon-IL10 familyRecombinant Human Cytokines$70.00 – $1,000.00
Description
Accession
P01579
Source
Optimized DNA sequence encoding Human Interferon gamma mature chain was expressed in Escherichia Coli.
Molecular weight
Native Interferon gamma is generated by the proteolytic removal of the signal peptide , the molecule has a calculated molecular mass of approximately 17 kDa. Recombinant Interferon gamma is a monomer protein consisting of 144 amino acid residue subunits, and migrates as an approximately 17 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>98%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by a viral resistance assay of mouse L929 cells and was found to be ≤ ng/ml, corresponding to a specific activity of ≥1 x units/mg.
Protein Sequence
MKYTSYILAF QLCIVLGSLG CYCQDPYVKE AENLKKYFNA GHSDVADNGT LFLGILKNWK EESDRKIMQS QIVSFYFKLF KNFKDDQSIQ KSVETIKEDM NVKFFNSNKK KRDDFEKLTN YSVTDLNVQR KAIHELIQVM AELSPAAKTG KRKRSQMLFR GRRASQ
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Interferon gamma was lyophilized from a 0.2μm filtered concentrated (1mg/ml) solution in PBS, pH.2.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Q66793
Interactor
Biological Process
Biological Process
Molecular function
Methods
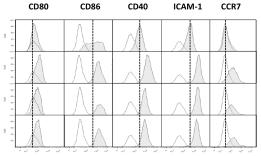
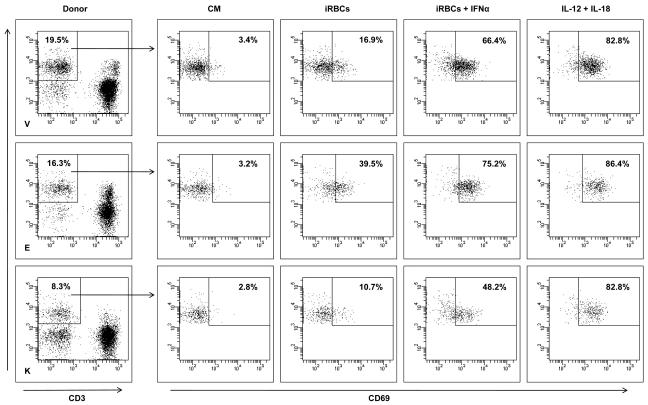
Day-2, Day-4 and Day-7 DCs pulsed with UVBL or FTL mature normally following LPS and IFN-γ stimulation.
- Following lysate loading, DCs were stimulated with LPS (60 EU/ml) and IFN-γ (2000 IU/ml) for 16 h. DCs were identified by HLA-DR, CD11c and one of the markers shown.
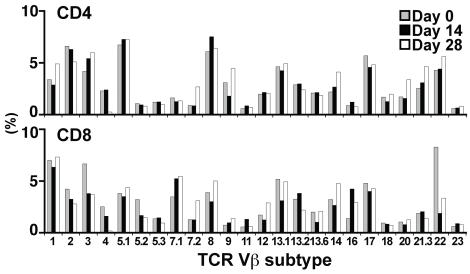
Expansion with aAPC/mOKT3 does not induce skewing of the TCR Vβ repertoire.
- Functional competence was demonstrated by antigen-specific cytotoxicity (middle) and IFN-γ secretion (right).
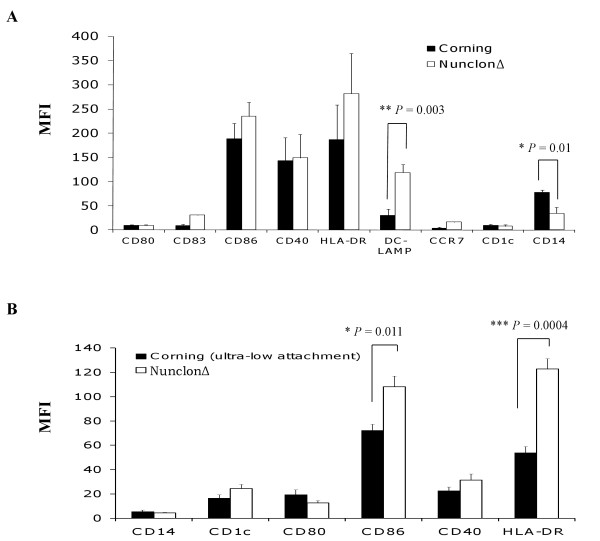
Comparison of the effect of Nunclon™Δ Surface, Corning.
- DC phenotype was evaluated following lysate-loading and maturation for 16 h with LPS and IFN-γ.
2.5. DC Maturation and Rapamycin Treatment
- Two days after monocytes were plated, monocyte-derived and hESC-derived immature DC were treated with 10 ng/mL and 5–7 ng/mL of rapamycin , respectively.
- On day 5, Cs were matured for 48 hr using a maturation cocktail consisting of 50 ng/mL of GM-CSF , 100 ng/mL IL-4 , 20 ng/mL IFN
γ , 50 ng/mL TNFα , 10 ng/mL of IL-1β , and 1μ g/mL PGE2 . - On day 6-7, DCs were harvested by gentle pipetting, passed through a 70
μ m cell strainer, centrifuged, and resuspended prior to their use in experiments.
Chemotaxis
- Chemotaxis assays were performed in 96-well transwell plates (Corning, Corning, NY, catalog #3388) using CCR5 or CCR1 transfectants.
- CCR5 and CCR1 expression vectors were made by amplifying the open reading frames from cDNA clones and inserting into pNEF38 5 transfectants per well.
- Chemokines (from) with or without inhibitors (MAb d5d7 antibody or controls) were added into the lower chambers.
- Migrating cells were harvested after 2 h and quantitated using CellTiter-Glo Luminescent Cell Viability Assay .
- Control inhibitors included vCCI-Fc (produced at VLST – the Fc is from human IgG1 with mutations to prevent interactions with Fc receptors 6 cells/mL with LPS at 1 µg/mL and recombinant human IFNγ at 1000 U/mL in the presence of 50 U/mL IL-2 for 48 h. The levels of CCL3, CCL4, and CCL5 in the supernatant were quantitated by Rules Based Medicine and found to be at 71 ng/mL, 398 ng/mL, and 1.7 ng/mL,…
Cell Migration Assay
- Cells were seeded at 106 cells/mL in 6-well plates with medium'>serum-containing medium and stimulated with tumor necrosis factor α (TNFα) (20 ng/mL) and IFNγ (500 units/mL) .
- After overnight incubation, medium was replaced with medium'>serum-free medium for 24 h. The conditioned supernatants were collected
Cell culture
- Peripheral blood monocytes and macrophages were collected from premenopausal women undergoing apheresis.
- Collection of patient samples was performed in accordance with the Helsinki Declaration under the guidelines of the National Cancer Institute Review Board, protocol number 99-CC-0168.
- Written informed consent was obtained from all human subjects as specified in the protocol.
- Monocytes and macrophages were separated from other cells using Ficoll-Hypaque gradient separation and selection by adherence to tissue culture plastic.
- Cells were grown in RPMI containing 5% human serum for 24 hr then changed to RPMI containing 5% FBS until differentiation.
- Differentiation was performed via treatment with 20 ng/ml of IFNγ and Lipopolysaccharide for 5 days.
Assay in U2OS cells
- To determine the repressive activity of GR ligands, cell line U2OS GR.G9, the human osteoblastic cell line U2OS, stably transfected with human GR, was used.
- Cells (104 cells/well) were incubated in 384-wells plates, in the presence of 50 ng/ml TNFα (&systems)/100 ng/ml IFNγ and compound, for 18 hours.
- Hereafter, a mixture of two different antibodies to hMCP-1, one Eu-labeled and one APC-labeled, were added.
- One hour later the time-resolved fluorometric resonance energy transfer (TR-FRET) signal was measured.
- (for more detailed information see Supportive Information) .
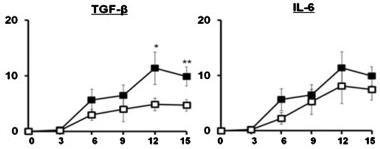
Exogenous T2-type cytokines, IL-10 and TGF-β, have inhibitory effects on HIV-1 replication in HCs.
- HCs were left untreated or pretreated for with IL-10, TGF-β, TNF-α, IFN-γ, IL-4 and IL-6 prior to infection with HIV-1BaL.
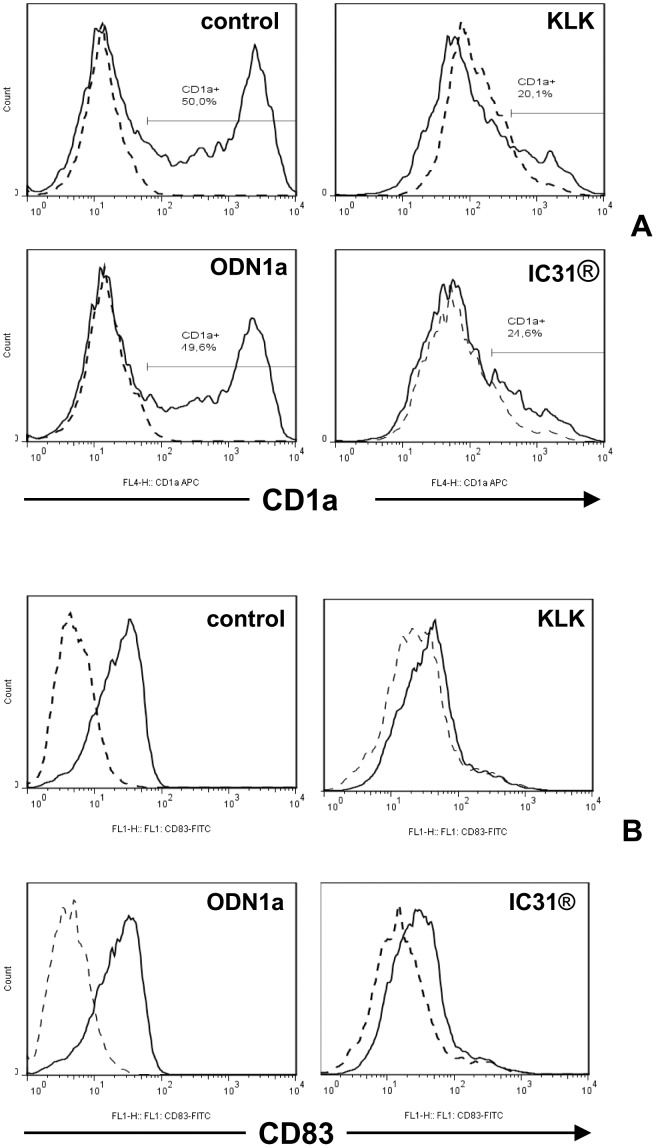
The effect of KLK, ODN1a and IC31® on the differentiation and activation of monocyte-derived dendritic cell subsets.
- B – Human moDCs were differentiated as described in Figure 2A and on day 5 they were activated by 100 ng/ml LPS +10 ng/ml IFNγ in combination with KLK, ODN1a and IC31® for 24 h (Protocol B, Figure S2).