Human Interferon-beta 1b Recombinant
Categories: Interferon-IL10 familyRecombinant Human Cytokines$70.00 – $160.00
Description
Accession
P01574
Source
Optimized DNA sequence encoding Human beta Interferon 1b mature chain was expressed in Chinese Hamster Ovary cells.
Molecular weight
Native Human Interferon-beta, generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calculated molecular mass of approximately 23 kDa. Recombinant Interferon-beta 1b is a monomeric protein consisting of 166 amino acid residue subunits, and migrates due to glycosylation as an approximately 23 kDa protein under non-reducing conditions and reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
Theactivity was determined by the cytopathic inhibition assay of human WISH cells infected with the ECMV virus, and determined to be 1x107 IU/mg.
Protein Sequence
MTNKCLLQIA LLLCFSTTAL SMSYNLLGFL QRSSNFQCQK LLWQLNGRLE YCLKDRMNFD IPEEIKQLQQ FQKEDAALTI YEMLQNIFAI FRQDSSSTGW NETIVENLLA NVYHQINHLK TVLEEKLEKE DFTRGKLMSS LHLKRYYGRI LHYLKAKEYS HCAWTIVRVE ILRNFYFINR LTGYLRN
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Interferon betabwas lyophilized from a 0.2 μm filtered solution containingmM NaOAc pH.5..
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
P17181
Interactor
Biological Process
Molecular function
Methods
Islet isolation, culture, and exposure to different beta-cell inhibitory substances
- Following the initial culture period, islets were cultured for an additional 6–24 hours in CMRL 1066 containing antibiotics, 2 mM glutamine and one of the following supplements: 0.5 mM sodium palmitate solubilized in 0.5% (weight/volume) fatty acid and lipopolysaccaride free bovine serum albumin (BSA) ; recombinant human Interleukin 1beta (IL-1ß (50 units/ml) and Interferon-gamma (IFN-γ) (1,000 units/ml) ; 100 mM hydrogen peroxide; 2 mM DETA/NO or 10 mM streptozotocin (STZ) .
- To some of the groups 10 µM of Imatinib was added at different time points prior to the addition of test substances given above.
- To controls equal amounts of vehicle (DMSO) were supplemented.
Western blotting for NF-κB, IκB-α and Smad7
- Interferon gamma (IFN-γ) 50 μl (100 ng/ml) was added to each dish in the experimental studies.
- The cytoplasmic and nuclear extracts were washed with ice-cold PBS and lysed in a 0.5 ml/well lysis buffer (150 mmol/l NaCl, 20 mmol/l Tris, pH 7.5, 0.1% Triton X-100, 1 mmol/l phenylmethylsulfonyl fluoride [PMSF] and 10 μg/ml aprotonin) as modified from the reports of Kim et al.
- and Moon et al.
- [
Replicative control of HCV in JFH-1/Huh7.5.1 system with conditioned media (CM) from pU/UC-transfected pDC-GEN2.2 cells.
- E) Recombinant Type III Interferons in the absence of CM at the same concentrations as found in the CM (IL-28A/IFNλ2: 1500 pg/mL; IL-28B/IFNλ3: 10 pg/mL; IL-29/IFNλ1: 500 pg/mL) were added to JFH-1 infected Huh7.5.1 cells.
CIK induction and intravenous infusion
- Venous blood was collected prior to chemotherapy or at least one month following chemotherapy.
- For the offspring (HLA haploidentical donors), the routine blood tests, and liver and kidney function tests were normal and negative for hepatitis A virus-IgM, hepatitis B surface antigen antibody , hepatitis B e --hepatitis B core-hepatitis C virus (HCV)-HCV-IgG, Syphilis-and human immunodeficiency virus-.
- As reported in our previous study (2 atmosphere.
- On the day of culture, 1,000 U/ml human recombinant interferon-γ , 500 U/ml recombinant human interleukin (rhIL)-1α and 1,000 U/ml rhIL-2 (Quangang Pharmaceutical Co., , , ) were added.
- Four days later, 1,000 U/ml rhIL-2 was added and the cells were transferred into a GT-T610 culture bag .
- Cell growth was observed every other day and cells were stained with 0.4% trypan blue and the viable cells were counted.
- Following 18 days of culture, cells were infused once daily (>1×109 cells; viability rate, >95%).
- A cycle of treatment…
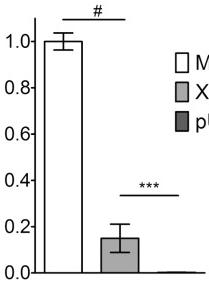
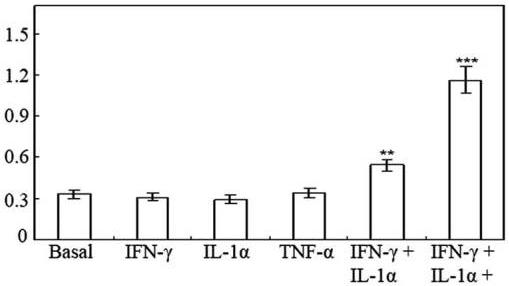
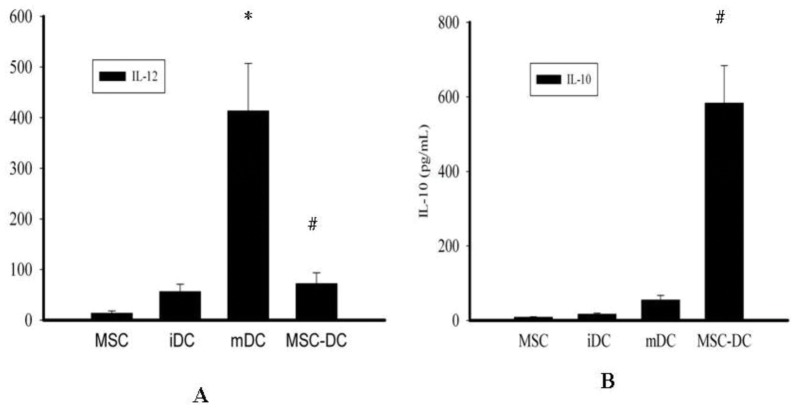
(A) Nitrite production by HT-29 cells following 48 h of treatment with cytokines.
- Effect of 0–50 ng/ml TNF-α on nitrite production induced by 100 U/ml IFN-γ and 10 ng/ml IL-1α in HT-29 cells following 48 h of treatment.
Invasion assay
- Cell migration through Matrigel-coated filters was measured using Transwell chambers with 8 μm-pore polycarbonate filters coated with Matrigel matrix as previously described (4 cells/well in the upper compartment of each invasion chamber and incubated for 24 h in the absence or presence of 100 U/ml interferon (IFN)-γ , 10 ng/ml interleukin (IL)1-α or 25 ng/ml tumor necrosis factor (TNF)-α , plus extract of Cnidiihizoma (0.1–5 mg/ml) or 1400W (0.5 mM).
- Non-migrating cells on the upper surface of the membrane were gently scrubbed with a cotton swab, and the invading cells on the lower surface were fixed with 100% methanol and stained with hematoxylin and eosin Y solution (RICCA Chemical Company, Charlotte, NC, USA).
- The number of cells was counted under a microscope at a magnification of ×100.