Human Epidermal Growth Factor Recombinant
Categories: EGF familyEGF familyRecombinant Human Cytokines$70.00 – $220.00
Description
Accession
P01133
Source
Optimized DNA sequence encoding Human EGF mature chain was expressed in Escherichia Coli.
Molecular weight
Recombinant Epidermal Growth Factor is a monomer protein consisting of 54 amino acid residue subunits, migrates as an approximately 6 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>98%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED50 was determined by a cell proliferation assay using balb/c 3T3 cells is ≤ 0.5 ng/ml, corresponding to a specific activity of ≥2 x 10^7 units/mg.
Protein Sequence
NSDSECPLSH DGYCLHDGVC MYIEALDKYA CNCVVGYIGE RCQYRDLKWW ELR
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
RecombinantEpidermal Growth Factor was lyophilized from a 0.2μm filtered concentrated (1mg/ml) solution in PBS, pH 7.2.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least 2 years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at 2° - 8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Molecular function
Methods
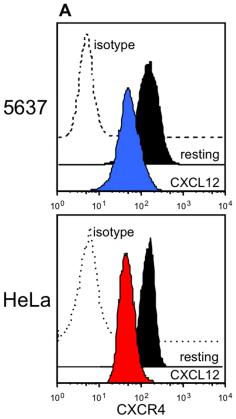
Receptors involved in the crosstalk between CXCL12 and HER1.
- 5637 or HeLa cells were cultured alone, or in the presence of 200 ng/mL CXCL12 or 25 ng/mL HB-EGF.
Enrichment, clonal expansion and redifferentiation of lung CSCs
- CSCs were enriched from CMT167 and LLC cells by culturing 10,000 cells/ml in medium'>medium'>serum-free medium'>medium'>MEM-F12 medium'>medium containing 50 μg/ml insulin , 0.4% Albumin Bovine Fraction V , N-2 Plus Media Supplement , B-27 Supplement , 20 μg/ml EGF and 10 μg/ml bFGF medium'>(CSC medium'>medium) in ultra-low attachment flasks to support growth of undifferentiated oncospheres.
- Oncosphere cultures were expanded by trypsinization and mechanical dissociation followed by re-plating of single cell suspensions (10,000 cells /ml) in fresh CSC medium.
- Oncospheres were collected for experiments after 2 weeks in non-adherent culture.
- For clonal expansion, single cells were added to each well of 96-well ultra-low attachment tissue culture plates (Corning) and clonal expansion was monitored at the indicated time points.
- Oncosphere diameters were determined using Image-Pro Plus 6.3 .
- To redifferentiate oncospheres, single cell suspensions were…
Cell culture, transfection and ES cell differentiation
- Differentiation assays were conducted as described 2 in Differentiation medium (GMEM, 10% KSR, 0.1 mM 2-mercaptoethanol, 1× non-essential amino acids and 1× sodium pyruvate).
- When indicated, Differentiation medium was switched to standard Neural Stem Cell (NSC) medium (DMEM/F12 , 2 mM L-glutamine, 0.6% glucose, 9.6 µg/ml putrescine, 6.3 ng/ml progesterone, 5.2 ng/ml sodium selenite, 25 µg/ml insulin, 0.1 mg/ml Apo-t-transferrin, 2 µg/ml heparin (sodium salt, grade II), all from), 10 ng/ml hEGF and 20 ng/ml hFGF2 .
- NSC medium was changed every 4 days.
- Where indicated, 1 µM all-trans retinoic acid , 13 ng/ml BMP4 and 4 µM γ-Secretase inhibitor were used.
- Time-lapse microscopy was carried out using the IncuCyte Live-Cell Imaging System .
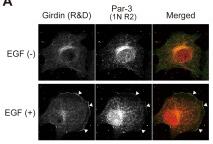
Colocalization of Girdin with Par-3 and the involvement of Girdin in random haptotoctic migration.
- Lysates from Vero cells, either stimulated for 30 min or non-stimulated with EGF (100 ng/mL), were immunoprecipitated with anti-Girdin and normal rabbit IgG antibodies, followed by Western blot analysis using the indicated antibodies.
Brain tumour stem cell culture
- To induce the growth and expansion of BTSCs as gliospheres, we adopted a culture condition established in our laboratory (−1 EGF and bFGF .
- The BTSCs (5 × 104 ml per well) were cultured in 12-well plates in 5% CO2 incubator at 37 oC, and 5 to 7-day-old gliospheres were used for the experiments.
Tissue Dissociation and Culture
- Biopsy human brain tissue containing the anterior temporal lobe and the hippocampus were obtained from surgery for medically refractory epilepsy.
- All specimens were collected with written patient consent and ethical approval from the Northern X Ethics Committee (biopsy tissue) and the University of Auckland Human Participants Ethics Committee.
- Eleven biopsy specimens (mean age 45 years) were collected from the surgical theatre at the Auckland City Hospital and transported to our laboratory in Ca2+ and Mg2+ free ice-cold Hank’s balanced salt solution (HBSS).
- Tissue containing the periventricular zone and the hippocampus was dissected and dissociated prior to being digested in HBSS containing 2.5 U/mL papain and 100 U/mL DNase 1 for 20 minutes at 37°C with gentle rotation, which included a gentle trituration step at 10 minutes.
- Enzymatic digestion was halted by the addition of NPC proliferation media; DMEM:F12 containing B27 , Penicillin/Streptomycin , GlutaMAX , 40 ng/mL FGF-2 ,…
2.3. Human ESC/iPSC Differentiation
- Human iPSCs were induced to form neural rosettes by culturing EBs for 7 days in suspension culture in medium composed of 50% medium'>DMEM/F12, 50% medium'>Neurobasal medium supplemented with glutamax, 0.5x N2 supplement, 0.5x B27 supplement , 0.5 mM ascorbic acid, 0.1% albumin, 4.5 × 10−4 M MTG , and bFGF (20 ng/mL).
- On the 7th day, EBs were plated onto gelatin-coated plates in the medium described above supplemented with EGF (20 ng/mL) and allowed to differentiate for an additional 4 days.
- On day 11, the number of EBs containing ≥1 neural rosette was manually counted.
Tumor Samples and Cell Culture
-
The GB cell lines were established from fresh GBM surgical specimens collected directly from the operating theatre in PBS plus 0,6% glucose and immediately transported on ice to the cell culture room and processed and cultured as monolayer cultures as previously described
in vitro assays of self-renewal, differentiation potential andin vivo tumor formation and propagation. - In our analysis were included also other GB lines (G166 and G179 cells) and GBM neurospheres (NSGBnR1) previously described in other studies 4 cells/well density in proliferation medium and subjected to lentiviral transduction
Mammosphere Formation Assay
- Mammosphere formation assay was performed as described previously 3–1×104 cells per milliliter single-cell suspensions were seed into six-well non-adherent plates in serum-free DMEM/F12 supplemented with 2% B27 , 20 ng/ml EGF , 20 ng/ml bFGF , 0.4% BSA , and 5 µg/ml insulin.
- After 7 day of culture, the numbers of mammospheres were counted.
Cell Culture
- HCT116 and HT29 colon cancer cell lines were maintained in DMEM/F12 supplemented with 10% FBS, 200 U/ml penicillin, 200 µg/ml streptomycin.
- Tumorsphere media (also called as serum free medium, SFM) was composed of DMEM/F12 media supplemented with 1×B27 , EGF (20 ng/ml), bFGF (10 ng/ml), routine insulin (5 µg/ml), 200 U/ml penicillin, and 200 µg/ml streptomycin.
- For 3D suspension culture, HCT116 and HT29 cells grown in two dimensional monolayer were digested with trypsin, resuspended, and then seeded at a density of 2×106 cells in SFM in 100 mm ultra-low attachment dishes (Corning) at 37°C in a humidified 5% CO2 ?
- 95% air atmosphere.