Human Epidermal Growth Factor Recombinant
Categories: EGF familyEGF familyRecombinant Human Cytokines$70.00 – $220.00
Description
Accession
P01133
Source
Optimized DNA sequence encoding Human EGF mature chain was expressed in Escherichia Coli.
Molecular weight
Recombinant Epidermal Growth Factor is a monomer protein consisting of 54 amino acid residue subunits, migrates as an approximately 6 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>98%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED50 was determined by a cell proliferation assay using balb/c 3T3 cells is ≤ 0.5 ng/ml, corresponding to a specific activity of ≥2 x 10^7 units/mg.
Protein Sequence
NSDSECPLSH DGYCLHDGVC MYIEALDKYA CNCVVGYIGE RCQYRDLKWW ELR
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
RecombinantEpidermal Growth Factor was lyophilized from a 0.2μm filtered concentrated (1mg/ml) solution in PBS, pH 7.2.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least 2 years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at 2° - 8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Molecular function
Methods
Identification of an ERK-aconitase protein complex that is disrupted by aconitase inhibition.
- mAcon-FLAG was coexpressed with ERK2 WT or D319N in HEK293T cells ±500 µM FA, ±100 ng/ml EGF, followed by IP and IB as in panel A.
Neural progenitor cell isolation and maintenance
- All procedures were performed in sterile fashion in a class II biosafety cabinet.
- A representative portion (2.5 cm) of three regions (thoracic, cervical and lumbar) of the spinal cord was used for Neural Progenitor Cell (NPC) isolation as described previously.
- Briefly, tissue was diced and a single cell suspension was obtained by enzymatic dissociation of the tissue at 37 °C for approximately 30–40 minutes with 2.5 U/ml papain , 250 U/ml of DNase I , and 1 U/ml neutral protease .
- After dissociation, the cell suspension was mixed with DMEM/F12 with 10% fetal bovine serum (FBS, , ), passed through a 70 μM filter, and centrifuged.
- The cell pellet was resuspended in DMEM/F12 with 10% FBS and combined 1:1 with percoll (GE Healthcare, Piscataway, NJ).
- The cell/percoll mixture was centrifuged at 20,000 g for 30 min at room temperature and the low buoyancy fraction (10 ml) above the red blood cell layer…
Isolation of epidermal stem cells and cell expansion
- Human foreskin samples were derived from voluntary circumcisions with informed consents and the protocol was approved by the Ethical Committee of the Institute of Zoology, Chinese Academy of.
- Human epidermal stem cells (hEpSCs) were isolated and expanded using our previous described method
Cell lines
- 081110 and 081024 non-adherent neurosphere lines were produced in our laboratory from human tumor samples received immediately following resection from consented GBM patients.
- Neurospheres were cultured in NeuroCult NS-A basal medium supplemented with 10ng/mL FGF , 10ng/mL EGF , and 0.0004% heparin .
- HCT116 cells and HCT116 lines containing only the wild-type (HPIK3CA WT #123) or mutant (HPIK3CA MT #125)
PIK3CA alleles were obtained from Dr. Bert Vogelstein (Johns Hopkins), and were grown in McCoy's Modified 5A medium (Sigma-Aldrich) supplemented with 10% FBS. - D54 and SKMG26 cells were grown in DMEM supplemented with 10% FBS.
Cell culture
- GM97, GM1600, and GM1605 were established from surgically resected specimens from glioblastoma patients at the University of California Los Angeles, in accordance with protocols approved by the University of California Los Angeles Institutional Review Board, as previously described 2 cell culture incubator .
- Glioblastoma and medulloblastoma cells grown as sphere cultures were collected by gentle centrifugation (800×g, 5 min) and trypsinized with 0.05% TrypLESelect for 5 min by pipetting.
- Cells were washed twice with phosphate buffered saline (PBS), counted, and seeded at a density of 2000 cells per 200 µL into 96-well ultralow-attachment plates (Corning).
Cell culture and transfections
- NIH 3T3 cells and NIH3T3 cells expressing the KDR-PFGFRA (KP) fusion protein were obtained as previously described 2 at 37°C in medium'>Dulbecco's medium'>Modified medium'>Eagle's medium (ATCC: 30-2002) with 10% heat-inactivated calf serum .
- Tumor sphere (TS) cells were isolated from primary human glioblastomas by disassociation into single cell suspension using Accumax ( Cell , , ) as previously described.
- s were filtered through a 100 µm filter, and plated in NeuroCult NS-A proliferation media supplemented with EGF (20 ng/ml& , , ), FGF (10 ng/mlocky , ), and heparin (2 µg/ml , , ).
- Oligonucleotides were transfected to a final concentration of 100 nM using the HiPerFect Transfection Reagent according to the manufacturer's instructions.
- Imatinib/Gleevec treatment of KP NIH 3T3 cells were performed at 10 uM.
Cell culture and transfections
- NIH 3T3 cells and NIH3T3 cells expressing the KDR-PFGFRA (KP) fusion protein were obtained as previously described 2 at 37°C in medium'>Dulbecco's medium'>Modified medium'>Eagle's medium (ATCC: 30-2002) with 10% heat-inactivated calf serum .
- Tumor sphere (TS) cells were isolated from primary human glioblastomas by disassociation into single cell suspension using Accumax ( Cell , , ) as previously described.
- s were filtered through a 100 µm filter, and plated in NeuroCult NS-A proliferation media supplemented with EGF (20 ng/ml& , , ), FGF (10 ng/mlocky , ), and heparin (2 µg/ml , , ).
- Oligonucleotides were transfected to a final concentration of 100 nM using the HiPerFect Transfection Reagent according to the manufacturer's instructions.
- Imatinib/Gleevec treatment of KP NIH 3T3 cells were performed at 10 uM.
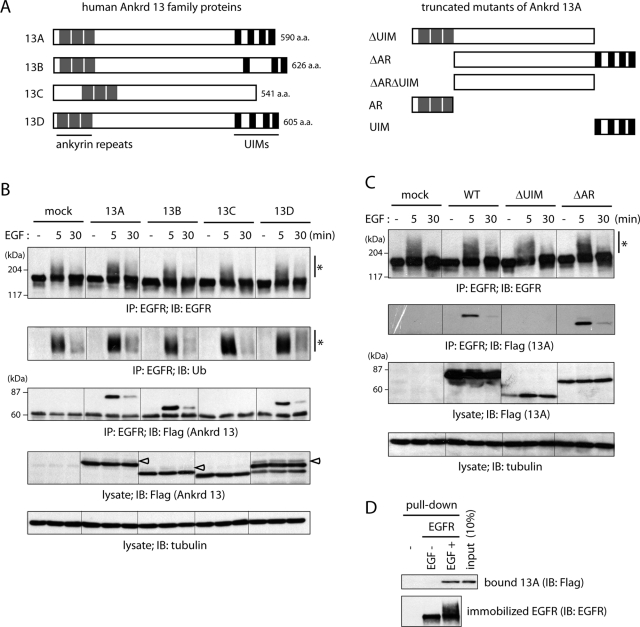
Ankrd 13A, 13B, and 13D bind to EGF-activated EGFR directly.
- HeLa cells were transfected with the indicated FLAG-Ankrd 13 constructs and treated with EGF for 5 or 30 min.
Isolation, and Culturing of Human Olfactory Bulb NSCs
- The OBs were harvested from adult patients undergoing craniotomy at the Institute of Neurosurgery, Catholic University, Rome (3 cells/cm2 in medium'>serum-free medium containing EGF and bFGF, and passaged up to P30.
- Between P7 and P10, parallel cultures were established in which cells were grown as adherent monolayers in medium containing EGF and bFGF supplemented with 5% fetal calf serum .
- Cells were counted with hemacytometer every 48 hours.
- Differentiation assays were performed by 14 days after plating on Matrigel coated glass coverslips in the absence of EGF and bFGF and in the presence of 1% fetal calf serum supplemented with 3′–5′-cyclic adenosine monophosphate (cAMP) 50 mM, all-trans retinoic acid 5 mM , and triiodothyronine (T3) 30 nM
Isolation, and Culturing of Human Olfactory Bulb NSCs
- The OBs were harvested from adult patients undergoing craniotomy at the Institute of Neurosurgery, Catholic University, Rome (3 cells/cm2 in medium'>serum-free medium containing EGF and bFGF, and passaged up to P30.
- Between P7 and P10, parallel cultures were established in which cells were grown as adherent monolayers in medium containing EGF and bFGF supplemented with 5% fetal calf serum .
- Cells were counted with hemacytometer every 48 hours.
- Differentiation assays were performed by 14 days after plating on Matrigel coated glass coverslips in the absence of EGF and bFGF and in the presence of 1% fetal calf serum supplemented with 3′–5′-cyclic adenosine monophosphate (cAMP) 50 mM, all-trans retinoic acid 5 mM , and triiodothyronine (T3) 30 nM