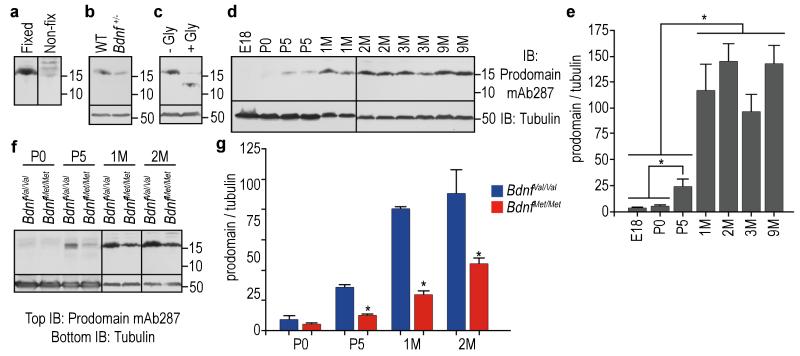
Human Brain-Derived Neurotrophic Factor Recombinant
Categories: Neurotrophic factorsRecombinant Human Cytokines$70.00 – $4,700.00
Description
Accession
P23560
Source
Optimized DNA sequence encoding Human Brain Derived Neurotrophic Factor mature chain was expressed in Escherichia Coli.
Molecular weight
Human native BDNF is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 14 kDa. Recombinant human BDNF is a disulfide-linked homodimeric protein consisting of two 119 amino acid residue subunits. migrates as an approximately 27 kDa protein under non-reducing conditions and as a 14 kDa protein under reducing conditions in SDS-PAGE.
Purity
>97%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent proliferation of rat C6 cells, and was found to be in the range of 0.5 ug/ml.
Protein Sequence
MTILFLTMVI SYFGCMKAAP MKEANIRGQG GLAYPGVRTH GTLESVNGPK AGSRGLTSLA DTFEHVIEEL LDEDQKVRPN EENNKDADLY TSRVMLSSQV PLEPPLLFLL EEYKNYLDAA NMSMRVRRHS DPARRGELSV CDSISEWVTA ADKKTAVDMS GGTVTVLEKV PVSKGQLKQY FYETKCNPMG YTKEGCRGID KRHWNSQCRT TQSYVRALTM DSKKRIGWRF IRIDTSCVCT LTIKRGR
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant Brain Derived Neurotrophic Factorwas lyophilized from a 0.2 μm filtered citric acid, pH.7.
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
Interactor
Interactor
Interactor
Q99523
Molecular function
Methods
Cell culture and cortical differentiation of human pluripotent stem cells
- The H1 and hESC line was cultured on Matrigel-coated cell culture plates (BD) in medium'>mTeSR-1 medium'>maintenance medium ( Cell ).
- In adherent conditions, hPSCs were seeded at a density of 5×104 cells/cm2 in growth medium.
- At 50% confluence, the medium was gradually changed to neural basal medium containing N2 and .
- SMAD signaling inhibitors LDN193189 (1 μM) and SB432542 ( 10 μM) were added from day 1 to day 7 of neural induction.
- Cyclopamine (400 ng/ml) and FGF-2 (10 ng/ml) were added from days 3-14 of differentiation.
- After 12-14 days, cells were mechanically passaged into poly-L-ornithine and laminin (20μg/ml) coated plates and allowed to undergo maturation for 3-6 weeks.
- BDNF (10 ng/ml) was added to cultures one week after initiation of neuronal maturation.
- For EB mediated neural differentiation, PSCs were aggregated for 4 days in ultra low-attachment plates (Corning) and then seeded on…
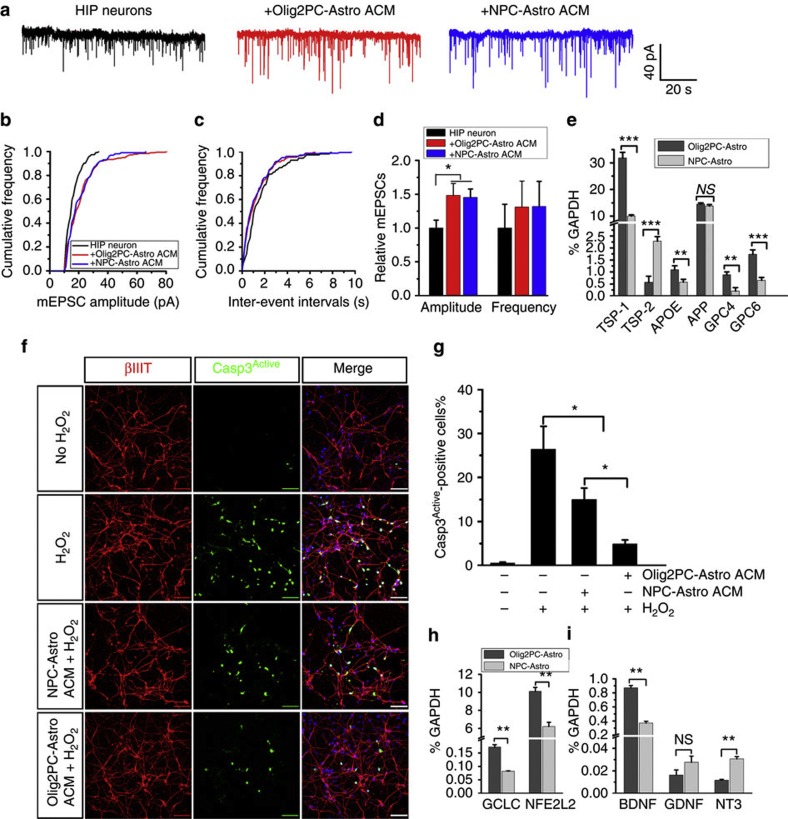
Effects of ACM from Olig2PC-Astros and NPC-Astros on neurons.
- (h and i) RT-PCR analysis of the expression of the antioxidant defense-related genes, GCLC and NFE2L2 (n=3), and the neurotrophic growth factor genes, BDNF, GDNF and NT-3 (n=3).
Differentiation of iNP cells.
-
Induced neural precursor colonies were generated by
SOX2/PAX6 plasmid transfection and cultured for 6 weeks in neural precursor proliferation media medium comprising of NBA media, 2% B27, 1% D-glucose, 1% Penicillin/Streptomycin/Glutamine, 0.2% EGF, 0.08% FGF-2, 0.2%1μM VPA and 25ng/ml Midkine . - For neuronal differentiation, the cells were mechanically dissociated and transferred onto poly-ornithine/laminin-coated (Sigma-Aldrich/) surface following full colony formation and differentiated using a two-step protocol as previously described.[ Briefly, cells were cultured in NBA media supplemented with 250ng/ml SHH (&systems), 100ng/ml KK1 , 20ng/ml BNF and 10μM Y27632 .
- After 10 days, culture conditions were changed to NBA media containing 0.5mM dibutryl-cyclic AMP , 0.5μM VPA, 20ng/ml BDNF and 10μM Y27632.
Human motor neuron differentiation
- Human embryonic stem cells (hESCs, lines H9, passages 19 to 35) were used to generate neuroectodermal cells.
- Motor neuron induction was carried out as previously described .
- Briefly, hESC–derived neuroectodermal cells were treated with retinoic acid (0.1 μM) for caudalization for one week in a chemically defined neural medium (NM: DMEM/F12, nonessential amino acids, 2 μg/ml heparin, and the neural cell supplement N2 .
- The neuroepithelial clusters were isolated and suspended in the NM in the presence of both retinoic acid and purmorphamine (1 μM).
- Purmorphamine was removed from the NM one week later.
- The formed progenitor spheres were subsequently cultured on glass coverslips coated with polyornithine and laminin (2–4 clusters/coverslip in a 24-well plate) in the presence of 0.5 ml NM, supplemented with BNF (10 ng/ml), GNF(10 ng/ml& ), IGF1 (10 ng/ml), cAMP (1 μM), ascorbic acid (200 ng/ml, Cell ), and 50 nM…
Human motor neuron differentiation
- Human embryonic stem cells (hESCs, lines H9, passages 19 to 35) were used to generate neuroectodermal cells.
- Motor neuron induction was carried out as previously described .
- Briefly, hESC–derived neuroectodermal cells were treated with retinoic acid (0.1 μM) for caudalization for one week in a chemically defined neural medium (NM: DMEM/F12, nonessential amino acids, 2 μg/ml heparin, and the neural cell supplement N2 .
- The neuroepithelial clusters were isolated and suspended in the NM in the presence of both retinoic acid and purmorphamine (1 μM).
- Purmorphamine was removed from the NM one week later.
- The formed progenitor spheres were subsequently cultured on glass coverslips coated with polyornithine and laminin (2–4 clusters/coverslip in a 24-well plate) in the presence of 0.5 ml NM, supplemented with BNF (10 ng/ml), GNF(10 ng/ml& ), IGF1 (10 ng/ml), cAMP (1 μM), ascorbic acid (200 ng/ml, Cell ), and 50 nM…
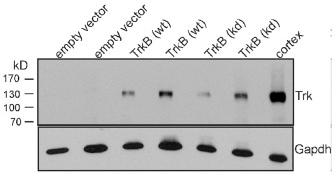
Expression of TrkB in human adenocarcinoma cells enhances cell motility in wound closure.
- Plates with serum-starved confluent cells were scratched with a pipette tip to create a single scratch wound, washed and cultured for another 24 h (A549 cells) or 20 h (NCI-H441 cells) in the absence or presence of BDNF (10 ng/ml).
Cell aggregation and migration assays
- For cell aggregation, cells were harvested from 80% confluent cultures with trypsin and plated at a density of 15.000 cells/cm2 on 9-cm dishes .
- After 24 h, the cultures were fed with new medium containing 0.5% foetal calf serum and further cultured for 24 h. Then, inhibitors were added 1 h prior to stimulation with growth factors, as indicated, and remained in the medium for the remainder of the experiment.
- BDNF or human EGF (10 ng/ml) were added for evaluation of cell-declustering and the culture was incubated for 24 h. Photographs were taken ( TS100 inverted microscope equipped with a 10x 0.24A phase contrast lens and a DS-2Mv camera).
- The number of cell clusters with a diameter larger than 125 µm was quantified; values are representative of at least 40 microscopic fields (area 0.7946 mm2 each) from three independent experiments.
Motor neuron culturing and differentiation
- iPSC were generated and characterized as described.
- Control fibroblasts were obtained from a 56-year-old man and a 40-year-old woman (ATCC), while mutant TDP43 fibroblasts were donated from a 56-year-old man.
- iPSCs were cultured on CF-1 irradiated mouse embryonic fibroblasts with KO-DMEM supplemented with 20% knockout serum replacement , 10 ng/ml basic FGF2 , 1 mM L-glutamine , 100 mM 2-mercaptoethanol , 1% penicillin/streptomycin and 1% nonessential amino acids.
- Before differentiation, cells were resuspended in MTESR1 media ( Cell ) and passaged three times.
- Neural precursor cells (NPCs) were generated by dual-SMAD inhibition in medium supplemented with SB431542 (10 μM), dorsomorphin (2.5 μM), and N-acetylcysteine (0.5 μM) for 5–7 days.
- Cells were dissociated with Accutase and caudalized using retinoic acid (0.1 μM) for 7–12 days, then dissociated again and plated onto coverslips coated with laminin and fibronectin in medium'>Neurobasal…
Motor neuron culturing and differentiation
- iPSC were generated and characterized as described.
- Control fibroblasts were obtained from a 56-year-old man and a 40-year-old woman (ATCC), while mutant TDP43 fibroblasts were donated from a 56-year-old man.
- iPSCs were cultured on CF-1 irradiated mouse embryonic fibroblasts with KO-DMEM supplemented with 20% knockout serum replacement , 10 ng/ml basic FGF2 , 1 mM L-glutamine , 100 mM 2-mercaptoethanol , 1% penicillin/streptomycin and 1% nonessential amino acids.
- Before differentiation, cells were resuspended in MTESR1 media ( Cell ) and passaged three times.
- Neural precursor cells (NPCs) were generated by dual-SMAD inhibition in medium supplemented with SB431542 (10 μM), dorsomorphin (2.5 μM), and N-acetylcysteine (0.5 μM) for 5–7 days.
- Cells were dissociated with Accutase and caudalized using retinoic acid (0.1 μM) for 7–12 days, then dissociated again and plated onto coverslips coated with laminin and fibronectin in medium'>Neurobasal…