Human Bone Morphogenetic protein-4 Recombinant
Categories: Recombinant Human CytokinesTGF-beta familyTGF-beta family$70.00 – $3,550.00
Description
Accession
P12644
Source
Optimized DNA sequence encoding Human Bone Morphogenetic protein-4 mature chain was expressed in Escherichia Coli.
Molecular weight
Recombinant BMP-4 is a homodimeric protein consisting of 2x106 amino acid residue subunits, and migrates as an approximately as a 12 kDa protein under reducing conditions in SDS-PAGE.
Purity
>95%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by its ability to induce alkaline phosphatase productionin mouse ATDC5 chondrogenic and was found to be in the range of 0.1-0.2 ug/ml.
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Recombinant BMP-4 was lyophilized from a 0.2 μm filtered PBS, pH 7.2
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at 2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
O88273
Interactor
Interactor
Interactor
Interactor
Q05438
Interactor
Biological Process
Biological Process
Biological Process
Molecular function
Molecular function
Molecular function
Methods
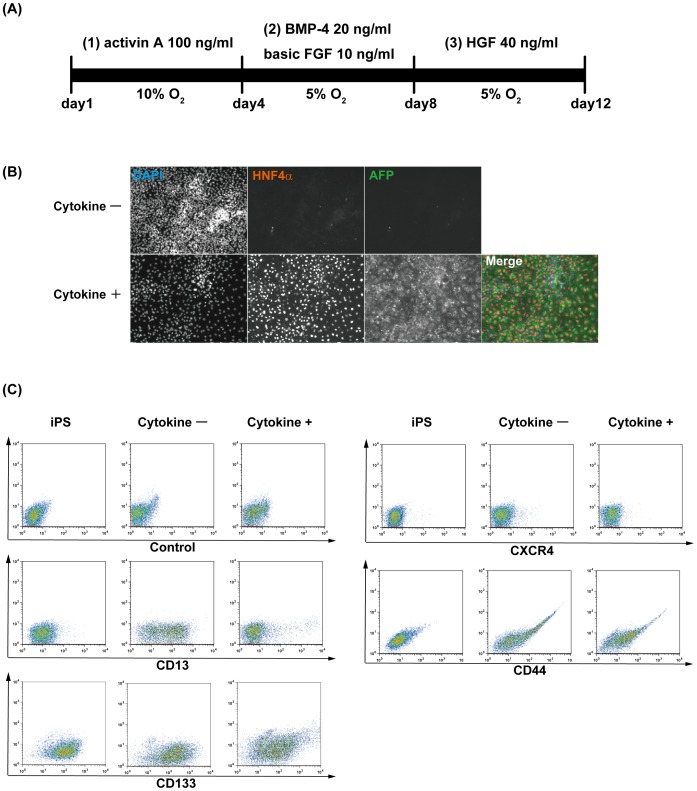
Differentiation from human iPS cells toward hepatic lineage cells.
- Human iPS cells were sequentially stimulated with various cytokines: (1) activin A, (2) basic FGF and BMP-4, and (3) HGF.
Human ES cell culture and differentiation
- Human H1, H9 and UCLA1-6 (UCLA stem cell core) [–1 bFGF .
- EBs were differentiated according to previous reports with minor modification [
μ M Y27632 (Rho kinase inhibitor IV, EMD Chemicals), 3 ng ml−1 Activin A , 5 ng ml−1 bFGF, 10 ng ml−1 BMP4 and 5μ M IWR-1 (EMD Chemicals), for the first 4 days. - EBs were then cultured in StemPro 34 supplemented with 10 ng ml−1 bFGF, 5 ng ml–1 vascular endothelial growth factor (VEGF) and 5
μ M IWR-1 for 5 additional days before plating onto elastic substrates. - Usage of all human ES cell lines is approved by the UCLA Embryonic Stem Cell Research Oversight (ESCRO) Committee and the Institutional Review Boards (IRB, approval #2009-006-04).
Induction of hESCs into primitive streak (PS) cells.
- (b) Comparison of expression levels of PS specific genes by combinatorial treatments with exogenous factors of Activin A, Wnt3a and BMP4, FGF2 , in hESC-derived PS cells.
Generation of CD34+ Cells from Embryoid Bodies (EB)
- We used an EB protocol described previously 2 incubator.
- On the next day, an equal amount of fresh medium'>EB medium with 10 ng/ml bFGF was added to the plate.
- After 3 days, cells were changed to medium'>EB medium with 10 ng/ml VEGF and 5 ng/ml bFGF.
- After 3 or 4 days additional culture, CD34+ cells were harvested by disrupting the EBs with collagenase IV/trypsin and affinity purified (MicroBead Kit ).
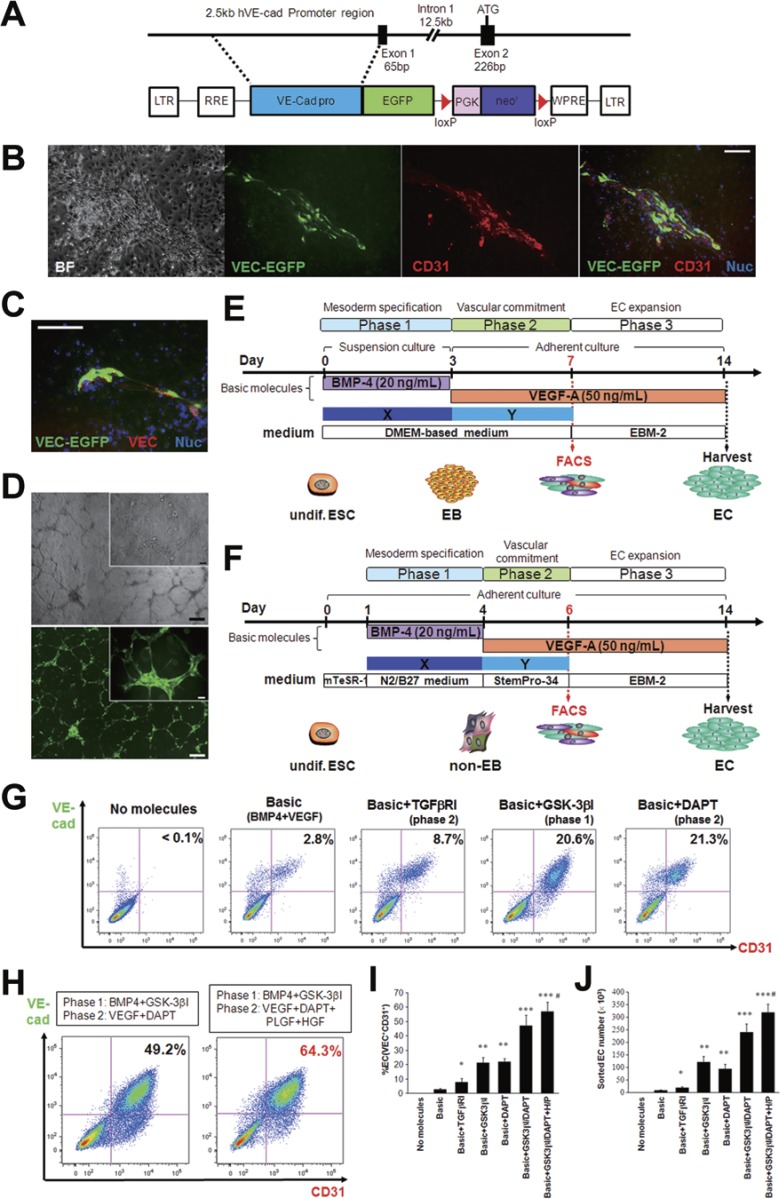
Establishment of a hESC reporter line for endothelial cell-specific lineage detection and the two modified protocols for endothelial differentiation.
- In method A , hESC-derived EBs were initially generated in suspension cultures with BMP4 (day 0-3; phase 1), and the EBs were transferred to adherent conditions on day 3 and cultured with VEGF-A (day 3-7; phase 2).
hiPSC differentiation
- hiPSCs were differentiated along a cardiac lineage as previously described 4 monothioglycerol , 50 µg/ml ascorbic acid , and 150 µg/ml transferrin .
- ifferentiation media was supplemented with 10 ng/ml BMP4 (ay 0).
- EBs were maintained in 6-well ultra-low attachment plates (Corning) at 37°C in 5% CO2, 5% O2, and 90% N2.
- On ay 1, media was changed to differentiation media supplemented with 10 ng/ml BMP4 and 15 ng/ml Activin A .
- On Day 4, media was changed to differentiation media supplemented with 1.5 µmol/L IWR-1
). - After Day 8, media was changed every 5 days to differentiation media without supplements.
hiPSC differentiation
- hiPSCs were differentiated along a cardiac lineage as previously described 4 monothioglycerol , 50 µg/ml ascorbic acid , and 150 µg/ml transferrin .
- ifferentiation media was supplemented with 10 ng/ml BMP4 (ay 0).
- EBs were maintained in 6-well ultra-low attachment plates (Corning) at 37°C in 5% CO2, 5% O2, and 90% N2.
- On ay 1, media was changed to differentiation media supplemented with 10 ng/ml BMP4 and 15 ng/ml Activin A .
- On Day 4, media was changed to differentiation media supplemented with 1.5 µmol/L IWR-1
). - After Day 8, media was changed every 5 days to differentiation media without supplements.
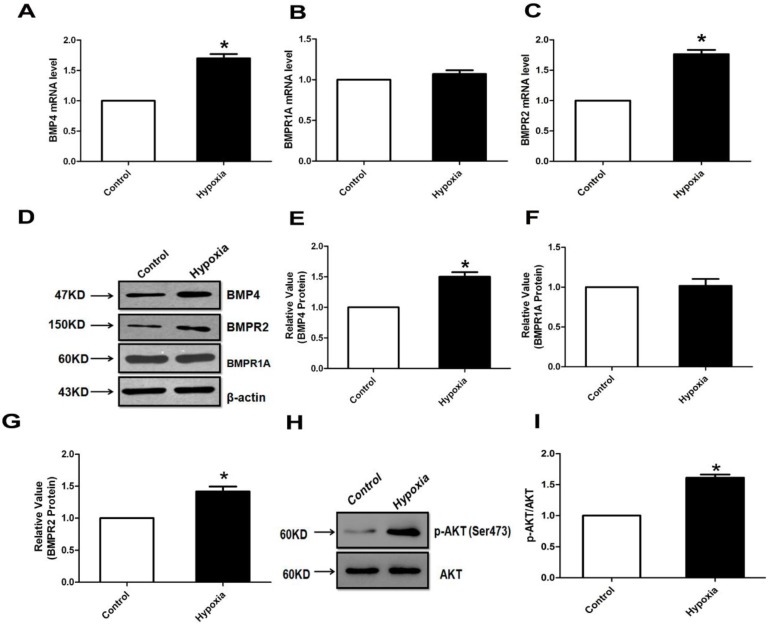
Bone morphogenetic protein 4 (BMP4) and its receptor (BMPR1A and BMPR2) mRNA and protein expression in pulmonary arteries.
- Quantitative real-time polymerase chain reaction (qPCR) was performed to analyze the BMP4 and its receptor (BMPR1A and BMPR2) expression.
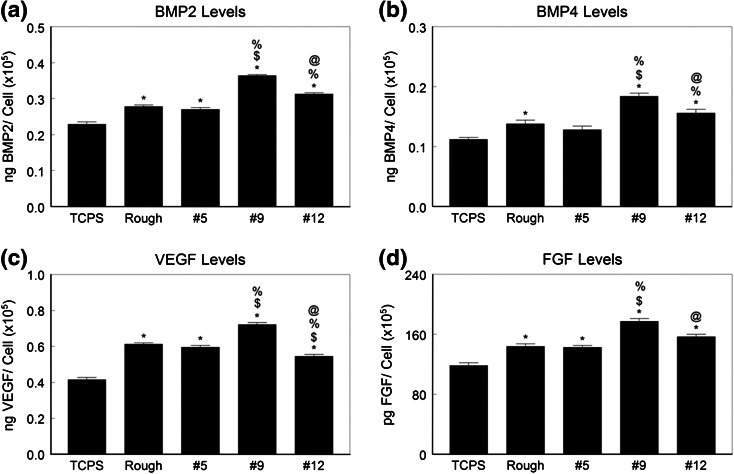
Local factor production by normal human osteoblasts on microstructured Ti6Al4V.
- Osteoblasts were cultured on TCPS, rTiAlV, #5, #9, or #12 surfaces and secretion of BMP2 (a), BMP4 (b), VEGFA (c), and FGF (d) measured in the conditioned media.
hPSC Differentiation
- Single-cell suspensions of hPSCs were obtained by treating the hPSC cultures at 80% confluency with 1× TrypLE .
- Single cells were plated at an optimized density ranging from 5,000 cells/cm2 to 15,000 cells/cm2 (depending on the cell line) onto six-well plates coated with 0.5 μg/cm2 of ColIV or 0.5 μg/cm2 TenC in medium'>E8 medium supplemented with 10 μM Rho kinase inhibitor .
- After 24 hr (day 0), the medium was changed to IF9S medium (see 2+ salt , 40 μl/l monothioglycerol , 8.4 μg/l additional sodium selenite , 10 g/l polyvinyl alcohol , 1× GlutaMAX , 1× nonessential amino acids , 0.1× chemically defined lipid concentrate , 10.6 mg/l Holo-Transferrin , and 20 mg/l insulin .
- Differentiation was conducted in a hypoxic condition from day 0 to day 5, and then in a normoxic condition from day 6 to day 9 (