Human beta Defensin-2 Recombinant
Categories: PDGF familyPDGF family$70.00 – $2,700.00
Description
Accession
O15263
Source
Optimized DNA sequence encoding Human beta Defensin-2 mature chain was expressed in Escherichia Coli.
Molecular weight
Native human BD-2 is generated by the proteolytic removal of the signal peptide and propeptide, the molecule has a calculated molecular mass of approximately 4 kDa. Recombinant beta Defensin-2 is a monomer protein consisting of 42 amino acid residue subunits, and migrates as an approximately 4 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>98%, as determined by SDS-PAGE and HPLC
Biological Activity
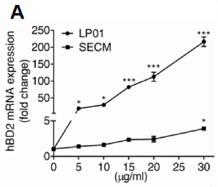
The ED(50) was determined by its ability to chemoattract immature human dendritic cells using a concentration range of 0-50 ng/ml.
Protein Sequence
MRVLYLLFSF LFIFLMPLPG VFGGIGDPVT CLKSGAICHP VFCPRRYKQI GTCGLPGTKC CKKP
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than.1 ng/µg(1EU/µg).
Presentation
Recombinant Human beta Defensin-2 was lyophilized with no additives
Reconstitution
A quick spin of the vial followed by reconstitution in 10mM Acetic acid to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at° -° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Molecular function
Methods
Histology & Immunohistochemistry
- Keratinocyte differentiation was analyzed using primary, antibodies directed against: Elafin (rabbit 92-1), hBD-2 (ab9871, ,), K10 (RKSE60, Eurodiagnostica) and K16 (LL025, Novocastra Laboratories, Newcastle upon Tyne,).
- Cell division was studied using antibodies against Ki67 (MiB-1, Dako cytomation).
- To enumerate CD4+ and CD8+ T cells antibodies against CD4 (BC/F6, Santa Cruz Biotechnology, Santa Cruz, CA) and CD8 (144B ) were used.
- IL-17 production was detected using polyclonal goat IL-17A antibody .
- To detect Foxp3 expression anti-FoxP3 (PCH101) was used.
- To detect the presence of human neutrophils and mast cells, antibodies against human neutrophil elastase (NP57) or human mast cell tryptase (AA1) were used.
- Antibody stainings were visualized using the EnVision+system-HRP kit combined with 3,3′-diaminobenzidine tetrahydrochloride or using that Labeled eptavidin Biotin method (LSAB Kit/AP) combined with either Permanent Red or 5-Bromo-4-Chloro-3-Indolyl Phosphate/Nitro Blue Tetrazolium (BCIP/NBT) .
Chemotaxis of Primary Lymphocytes
- Peripheral blood lymphocytes were resuspended in RPMI supplemented with 1% FBS at a density of two million cells per mL.
- Serial dilutions of recombinant human β-defensin 2 (hBD2, , , ) or equivalent vehicle control were prepared in the same media.
- hBD2 and vehicle dilutions were plated in a ChemoTX plate , alongside media alone controls.
- The ChemoTX filter was attached to the plate, and 50 µL lymphocyte suspension was applied to the surface of each well.
- The ChemoTX plate was incubated at 37°C/5% CO2 for 3 hr.
- To compare migrated cells, media above the filter was removed, and apical surface of filter was washed once in DPBS with 5 mM EDTA, then incubated with the same wash for 30 min at 4°C.
- This second wash was removed, the ChemoTX plate was centrifuged at 400×g for 5 min, and the filter was removed.
- Cells in the lower chamber were resuspended in a…
hBD2 Acid-Urea (AU) Western
- Epithelial cells were harvested and lysed by scraping into 10% acetic acid.
- These cell lysates were vortexed 30 min at room temperature to extract protein.
- Soluble extracts were clarified and concentrated, then resolved on an acid-urea polyacrylamide gel electrophoresis (AU-PAGE).
- A standard of recombinant hBD2 was run alongside cell extracts on each gel.
- Gels were transferred to PVDF membranes and blotted with a goat polyclonal antibody against hBD2 .