Human basic Fibroblast Growth Factor Recombinant
Categories: FGF familyFGF familyRecombinant Human Cytokines$70.00 – $700.00
Description
Accession
P09038
Source
Optimized DNA sequence encoding Human basic Fibroblast Growth Factor mature chain was expressed in Escherichia Coli.
Molecular weight
Recombinant bFGF is a monomer protein consisting of 155 amino acid residue subunits, and migrates as an approximately 17 kDa protein under non-reducing and reducing conditions in SDS-PAGE.
Purity
>96%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) was determined by the dose-dependent stimulation of thymidine uptake by BaF3 cells expressing FGF receptors, corresponding to a specific activity of ≥2x10^7 units/mg.
Protein Sequence
MAAGSITTLP ALPEDGGSGA FPPGHFKDPK RLYCKNGGFF LRIHPDGRVD GVREKSDPHI KLQLQAEERG VVSIKGVCAN RYLAMKEDGR LLASKCVTDE CFFFERLESN NYNTYRSRKY TSWYVALKRT GQYKLGSKTG PGQKAILFLP MSAKS
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Human Basic FGF was lyophilized from a 0.2 μm filtered solution inM NaCl,20mM PB pH7.0 .
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Interactor
O35082
Interactor
O43353
Interactor
Interactor
Interactor
Q802A9
Interactor
Interactor
Biological Process
Biological Process
Molecular function
Molecular function
Molecular function
Methods
Neural in vitro differentiation
-
Neural differentiation of LLC6P and LLC9P cells was performed as previously described
g for 5 minutes at 4°C and plated on polyornithine/laminin-coated cell culture dishes. - Passage number of LLC6P hpESCs at differentiation induction was 30–45; line LLC9P cells were used at passage numbers 48–60.
- Terminal differentiation of hpNSCs was performed in differentiation media containing DMEM/F12 (N2 supplement; 1∶50) and Neurobasal (B27 supplement; 1∶50) mixed at 1∶1 ratio.
- cAMP (300 ng/mL) was added to the media for 28 days.
- For induction of dopaminergic differentiation
Cell isolation and cultivation
- After washing the cord with ulbecco’s phosphate buffered saline (PBS) the vein was filled with 2.4 U/ml dispase II solution and incubated for 30 min in a humidified atmosphere of 37°C and 5 % CO2 for isolation of HUVEC according to conventional & .
- Under GMP conditions, 0.12 U/ml collagenase NB6 solution was injected into the vein and incubated for 20 min.
- In accordance with&conditions, collected HUVEC were cultured in Medium 199 supplemented with 10 % FCS , 1 % penicillin/streptomycin , 5 U/ml heparin and 10 ng/ml basic fibroblast growth factor (bFGF) at 37°C in a 5 % CO2 atmosphere.
- To achieve conditions conforming to GMP, Medium 199 was supplemented with 10 % HS (pooled AB serum for , ), 1 % gentamicin , 5 U/ml heparin (pharmaceutical grade, , ) and 10 ng/ml bFGF .
- The following day, the HUVEC were washed with PBS to remove all non-adherent cells.
- At confluence, the…
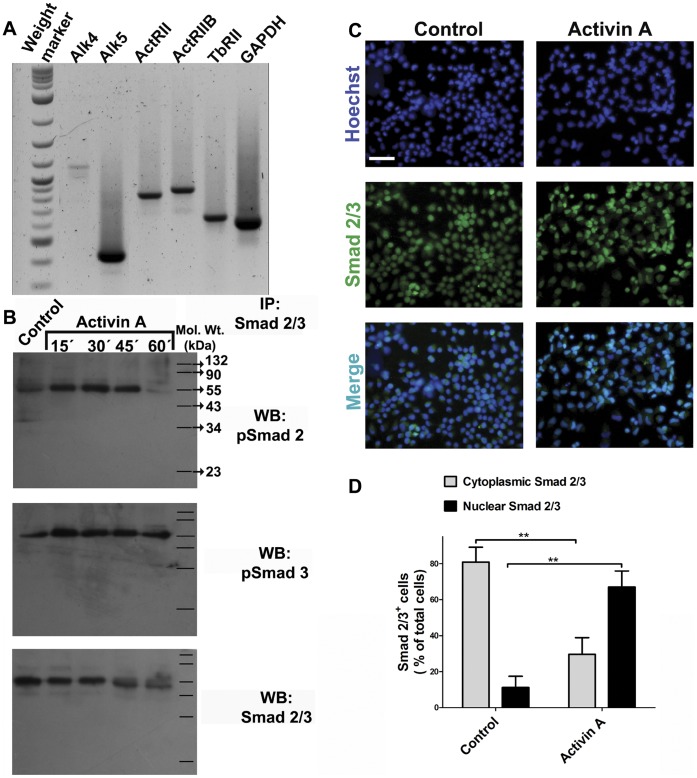
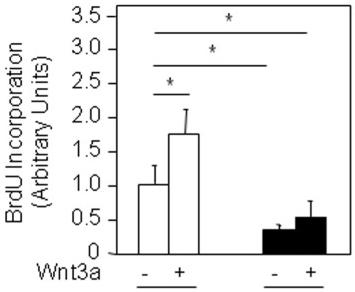
Cerebrocortical neural progenitor cells are responsive to addition of Activin A.
- Passage 2 neural progenitor cells were obtained from E14 rat embryos and kept in proliferation with FGF2.
Derivation and Culture of mEpiSC Lines
-
The mEpiSC lines were established following the procedure of Tesar
et al. −4 M 2-ME, 10 ng/mL activin A , and 10 ng/mL human recombinant FGF2 on mitomycin C-treated mouse embryonic fibroblasts. - Growing colonies were typically observed within a few days with differentiated cells.
- To purify stem cell colonies, differentiated cells surrounding the stem cells were discarded by manually picking under a microscope (until passage 5).
- Cells were routinely passaged on fibronectin -coated dishes every 3 days using accutase or 1 mg/mL collagenase type-IV dissolved in DMEM-F12 at a concentration of 10 mg/mL as a stock solution.
- Thereafter mEpiSCs were usually passaged with enzyme treatment as described above.
- After several passages (>5 passages on MEF feeder layer), mEpiSCs were usually stably self-renewing.
- mEpiSCs were adapted and maintained under feeder-free conditions as described previously
Cell culture
- GL261 glioma cells were cultured in DMEM-F12 Glutamax™-1 , B-27 supplement 1X , penicillin/streptomycin 1X, human recombinant epidermal growth factor (EGF; 20 ng/ml), and human recombinant fibroblast growth factor-2 (FGF-2; 20 ng/ml).
- The spleens and draining lymph nodes of immunized and control mice were surgically removed and mechanically processed in medium'>RPMI medium'>1640 basal medium (LONZA) containing 5% fetal bovine serum, 20 mM HEPES, 2% L-glutamine and penicillin/streptomycin.
- Erythrocytes were lysed with ice-cold ACK buffer, and lymphocytes were resuspended in RPMI 1640 supplemented with 10% fetal bovine serum, 2% penicillin/streptomycin, 2% L-glutamine, 50 μM β-mercaptoethanol, 100 mM sodium pyruvate and 100X nonessential amino acids.
- We also added 10 U/ml human recombinant IL-2 to the medium for all in vitro experiments.
Mammosphere culture conditions (MS-proficient condition) and Sphere-forming eficiency (SFE)
- Single-cell suspensions derived from monolayer cultures were maintained in a serum-free “stem medium” consisting of DMEM F-12 medium supplemented with 4 ug/ml heparin , 0,6% glucose, 0,1% NaHCO3, 100 ug/ml apotransferrin, 25 ug/ml insulin, 9 ug/ml putrescine (1–4 diaminobutane dihydrochloride) and 3 × 10–4 M sodium selenite in ultra-low attachment plates (Corning).
- Every other day, culture medium was enriched with fresh 20 ng/ml EGF and 10 ng/ml bFGF .
- Mammospheres were collected by centrifugation every 6–12 d and dissociated enzymatically (5 min in 0,05% Trypsin/EDTA) or mechanically by pipetting.
- The obtained single cells were re-plated for secondary and tertiary cultures and collected for RNA and protein.
Mammosphere culture conditions (MS-proficient condition) and Sphere-forming eficiency (SFE)
- Single-cell suspensions derived from monolayer cultures were maintained in a serum-free “stem medium” consisting of DMEM F-12 medium supplemented with 4 ug/ml heparin , 0,6% glucose, 0,1% NaHCO3, 100 ug/ml apotransferrin, 25 ug/ml insulin, 9 ug/ml putrescine (1–4 diaminobutane dihydrochloride) and 3 × 10–4 M sodium selenite in ultra-low attachment plates (Corning).
- Every other day, culture medium was enriched with fresh 20 ng/ml EGF and 10 ng/ml bFGF .
- Mammospheres were collected by centrifugation every 6–12 d and dissociated enzymatically (5 min in 0,05% Trypsin/EDTA) or mechanically by pipetting.
- The obtained single cells were re-plated for secondary and tertiary cultures and collected for RNA and protein.
Mammosphere culture
- Cells in the supernatant of 2-day-old cultures were collected by centrifugation, for 5 minutes at 300×g, washed in Hanks' buffered salt solution, and re-suspended in phenol red-free DMEM-F12 supplemented with 2% B27 , 5 µg/mL bovine insulin , 10 ng/mL bFGF , and 20 ng/mL epidermal growth factor at a density of 2,000 cells/mL to obtain cancer-initiating cells and propagate them as mammospheres.
- Growth factors were added to the mammosphere cultures (
Mammosphere culture
- Cells in the supernatant of 2-day-old cultures were collected by centrifugation, for 5 minutes at 300×g, washed in Hanks' buffered salt solution, and re-suspended in phenol red-free DMEM-F12 supplemented with 2% B27 , 5 µg/mL bovine insulin , 10 ng/mL bFGF , and 20 ng/mL epidermal growth factor at a density of 2,000 cells/mL to obtain cancer-initiating cells and propagate them as mammospheres.
- Growth factors were added to the mammosphere cultures (