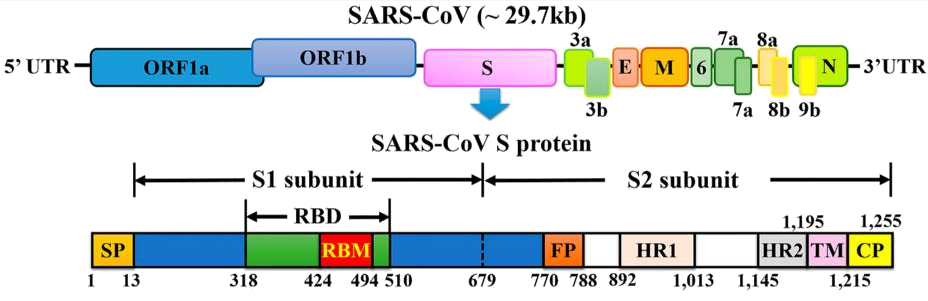
The envelope-anchored trimeric spike protein mediates coronavirus entry into host cells by binding to its host receptor and subsequently fusing host and viral membranes.
Coronavirus S proteins, including SARS-S, share several features: Peplomers of coronavirus S proteins protrude from the virion surface and are responsible for the corona-like shape of virions observed upon electron microscopy.
A hallmark of these proteins is the presence of an N-terminal surface unit (termed S1 in the context of CoV S proteins), which harbors the receptor binding domain and a C-terminal transmembrane unit (termed S2 in the context of CoV S proteins), which contains the functional elements required for membrane fusion: a fusion peptide, heptad repeats and a transmembrane domain.
A second characteristic shared by class I membrane fusion proteins is the transition of the transmembrane unit into a thermostable, protease-insensitive conformation termed six-helix-bundle upon successful completion of the membrane fusion reaction.he first indispensable step of the entry cascade is the binding of the S proteins to a receptor.
Upon activation, a fusion peptide located at the N-terminus of the S2 subunit inserts into a target cell membrane, either the plasma membrane or an endosomal membrane.
The receptor binding domain (RBD) of SARS-S is located at the C-terminus of the S1 subunit and binds to ACE2 with high affinity. The RBD consists of two subdomains, a core and an extended loop. The core structure is conserved between SARS-S and other CoV S proteins but is not directly involved in ACE2 binding.Whether a host is susceptible to SARS-CoV is largely determined by the binding affinity between SARS-CoV RBD and host ACE2 in the initial step of viral attachment
Upon activation, a fusion peptide located at the N-terminus of the S2 subunit inserts into a target cell membrane. The S2 subunit is connected with the viral membrane via its transmembrane domain and the cellular membrane via the fusion peptide
No products were found matching your selection.

