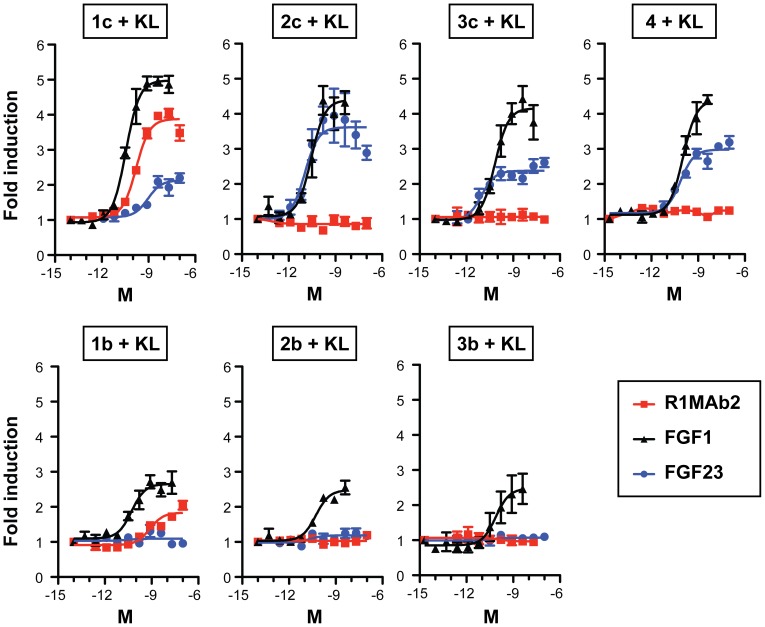
Rat basic Fibroblast Growth Factor Recombinant
Categories: FGF familyRecombinant Rat Cytokines$70.00 – $1,100.00
Description
Accession
P13109
Source
Optimized DNA sequence encoding Rat basic Fibroblast Growth Factor mature chain was expressed in Escherichia Coli.
Molecular weight
Native Rat FGF basic is generated by the proteolytic removal of the signal peptide and propeptide,the molecule has a calulated mass of kDa. Recombinant Rat bFGF is a monomer protein consisting of 154 amino acid residue subunits, and migrates as an approximately 16 kDa protein under reducing conditions in SDS-PAGE.
Purity
>96%, as determined by SDS-PAGE and HPLC
Biological Activity
The ED(50) determined by dose-dependent stimulation of thymidine uptake byT3 cells expressing FGF receptors, was ofund to be <1 ng/ml.
Protein Sequence
MAAGSITSLP ALPEDGGGAF PPGHFKDPKR LYCKNGGFFL RIHPDGRVDG VREKSDPHVK LQLQAEERGV VSIKGVCANR YLAMKEDGRL LASKCVTEEC FFFERLESNN YNTYRSRKYS SWYVALKRTG QYKLGSKTGP GQKAILFLPM SAKS
Endotoxin
Endotoxin content was assayed using a LAL gel clot method. Endotoxin level was found to be less than 0.1 ng/µg(1EU/µg).
Presentation
Rat Basic FGF was lyophilized from a 0.2 μm filtered PBS solution pH7.5 .
Reconstitution
A quick spin of the vial followed by reconstitution in distilled water to a concentration not less than 0.1 mg/mL. This solution can then be diluted into other buffers.
Storage
The lyophilized protein is stable for at least years from date of receipt at -20° C. Upon reconstitution, this cytokine can be stored in working aliquots at2° -8° C for one month, or at -20° C for six months, with a carrier protein without detectable loss of activity. Avoid repeated freeze/thaw cycles.
Usage
This cytokine product is for research purposes only.It may not be used for therapeutics or diagnostic purposes.
Biological Process
Biological Process
Molecular function
Molecular function
Molecular function
Methods
Time-lapse imaging assay
- Cells were transfected with fucci2;mAG-hGem(1/110), which labels nuclei in the S/G2/M phases 2/M marker were grown in 96-well dishes in serum-free DMEM HAM/F12 supplemented with 1×B27 , 20 ng/mL epidermal growth factor (EGF), and 20 ng/mL fibroblast growth factor-2 (FGF-2), at 37°C with 5% CO2.
- The cells were imaged for 96 hours in an incubator on the microscope stage.
- Fifty-one images in the z-axis, both bright-field and single-color (green), were captured at 20-min intervals.
- An inverted microscope (IX-71, Olympus) was fitted with a Nipkow disc scanning confocal unit (CSU10, Yokogawa Electric Corp.), EM-CCD camera (iXON BV-887, Andor), filter wheel, and z motor (Mac5000, Ludl Electronic Products).
- As our imaging device has an attached auto xy stage , several spheres can be monitored in one assay.
- Device control and image analysis were performed using MetaMorph software .
Cell culture
- NSCs isolated from the hippocampi of 6-week-old female Fisher 344 rats , were cultured as previously described on poly-ornithine/laminin-coated plates in medium'>DMEM/F12 medium containing N2 supplement and 20 ng/mL FGF-2 , with subculturing upon reaching 80% confluency using Accutase ( Flow ).
- To induce differentiation, NSCs were cultured for five days in DMEM/F12/N2 medium supplemented with 2% fetal bovine serum (FBS ) and 1 µM retinoic acid (BIOMOL).
- Rat hippocampal astrocytes were isolated from Fisher 344 rats as previously described and cultured on poly-ornithine/laminin-coated plates in DMEM/F12/N2 supplemented with 10% FBS, with subculture upon reaching 90% confluency using Trypsin EDTA .
-
Oligodendrocyte. Cells were seeded at a concentration of 5,000 cells/cm2 on tissue culture plastic plates and coverslips and cultured in high glucose DMEM supplemented with 1% Penicillin/Streptomycin , 2 mmol/lL -Glutamine , 1X N1 supplement , 1 µg/ml biotin , 5 ng/ml bFGF , 1 ng/ml PDGF , and 30% B104-conditioned media for 1 day. - On the second day, CG4 rat oligodendrocyte progenitor cells were added in a co-culture setting to promote differentiation, using co-culture membrane inserts, and the media were changed every 2 days.
- The cells were allowed to differentiate for 5 days and were then fixed and stored in PBS for immunofluorescence.
- Cells were subsequently assessed for expression of the oligodendrocyte markers O2 and NG2.
Cell isolation
- Cells were isolated from the blood vessels in rat, mouse and human.
- Human carotid arteries were obtained from .
- The cells isolation methods were described previously .
- Briefly, the tissue segments were washed three times with phosphate buffered saline (PBS) supplemented with 1% penicillin/streptomycin (P/S).
- The surrounding connective tissues and adventitia were dissected away under a dissecting microscope.
- Endothelium was removed by scraping off the cell layer on the luminal surface with sterile scalpel blades.
- For tissue explant culture method, the tunica media was cut into mm-size and placed onto the surface coated with 1% CellStart in 6-well plates.
- The cells were cultured in MEM with 10% FBS , or in MEM with 2% CEE (MP , ), 1% FBS, 1% N2 , 2% B27 , 100 nM retinoic acid (A) , 50 nM 2-mercaptoethanol (2ME) , 1% P/S and 20 ng/ml bFGF .
- For enzymatic digestion methods, tissues were…
Isolation of Skin Derived Progenitor Cells
- SKPs were isolated from 6 weeks old female Sprague Dawley rats as described previously 2 inhalation.
- Back skin was depilated using wax strips and a piece of skin was removed.
- Skin was washed in cold HBSS and fat layers were removed from the skin underside using forceps.
- Skin was then cut into small pieces using razor blades and digested with collagenase XI (1 mg/ml) /HBSS for 1 hr at 37°C.
- Collagenase/skin cell suspension was passed through a 70 µm mesh to remove remaining tissue pieces.
- Collagenase/cell suspension was diluted with DMEM+Glutamax and cells were isolated by centrifugation (5 min, 1200 rpm).
- Cells were plated in DMEM/F12 3∶1+ Glutamax medium containing 2% B27 , 20 ng/ml EGF , 40 ng/ml FGF2 , Penicillin/Streptomycin and Fungizone .
- Under these conditions, SKPs grow in suspension as spheres.
- For passaging, SKPs spheres were isolated from the culture medium by centrifugation (5 min, 1200 rpm)…
Isolation of Skin Derived Progenitor Cells
- SKPs were isolated from 6 weeks old female Sprague Dawley rats as described previously 2 inhalation.
- Back skin was depilated using wax strips and a piece of skin was removed.
- Skin was washed in cold HBSS and fat layers were removed from the skin underside using forceps.
- Skin was then cut into small pieces using razor blades and digested with collagenase XI (1 mg/ml) /HBSS for 1 hr at 37°C.
- Collagenase/skin cell suspension was passed through a 70 µm mesh to remove remaining tissue pieces.
- Collagenase/cell suspension was diluted with DMEM+Glutamax and cells were isolated by centrifugation (5 min, 1200 rpm).
- Cells were plated in DMEM/F12 3∶1+ Glutamax medium containing 2% B27 , 20 ng/ml EGF , 40 ng/ml FGF2 , Penicillin/Streptomycin and Fungizone .
- Under these conditions, SKPs grow in suspension as spheres.
- For passaging, SKPs spheres were isolated from the culture medium by centrifugation (5 min, 1200 rpm)…
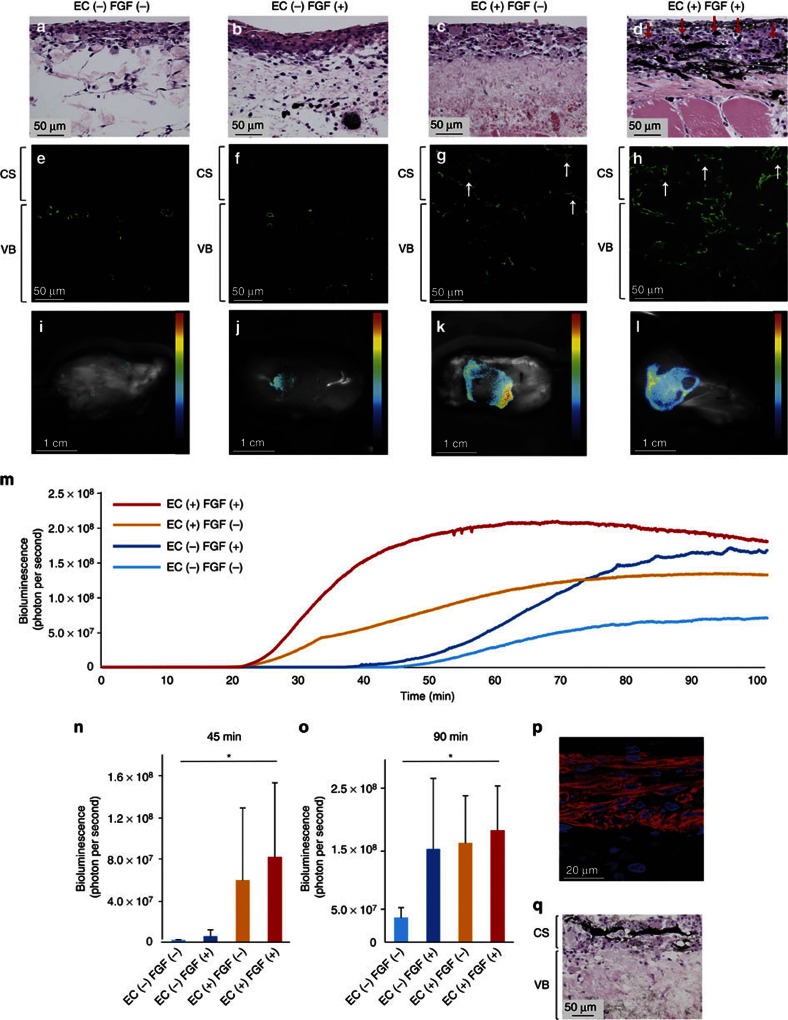
Perfusable blood vessel formation and viable cardiac tissue fabrication.
- Blood vessel formation and tissue viability were evaluated among the four groups, with and without EC coculture, and with or without FGF-2 administration in the perfusion medium.
Retrovirus infection and other in vivo manipulations
- High-titer solutions (2×108 colony forming unit/ml) of recombinant retroviruses pMXIG and its derivatives expressing Neurog2, Pax6, and Ascl1 were prepared with artificial cerebrospinal fluid (124 mM NaCl, 5 mM KCl, 1.3 mM MgCl2, 2 mM CaCl2, 26 mM NaHCO3, and 10 mM D-glucose, with the pH adjusted to 7.2 using aeration with 95% O2–5%CO2) containing rat serum albumin (1 mg/ml).
- In some experiments, the virus solution was supplemented with FGF2 (0.9 μg/ml) and EGF (0.9 μg/ml).
Preparation of SLCs
- After being subcultured at a concentration of 1 × 106 cells/cm2, BM-MSCs were incubated in αMEM containing 1 mM BME without serum for 24 h. The culture medium was then replaced with αMEM containing 10% FBS and 35 ng/ml at-RA .
- After three days, the cells were finally transferred to inducer medium containing αMEM, 10% FBS and trophic factors of 5 μM FSK , 10 ng/ml bFGF , 5 ng/ml PGF , and 200 ng/ml HG .
- The cells were cultured for 10 days [
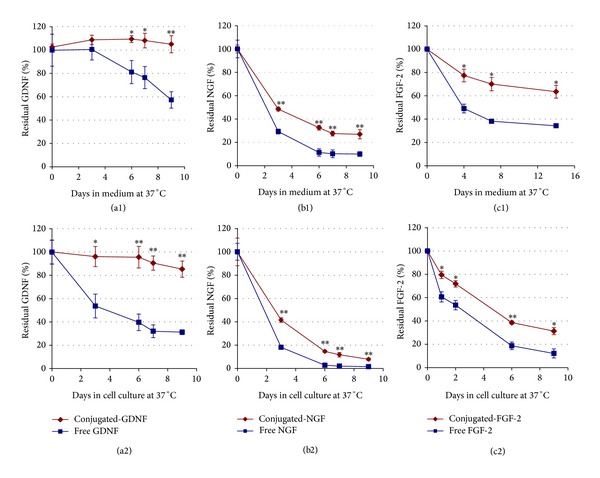
Stability of free versus conjugated neurotrophic factors at 37°C in the absence ((a1), (b1), and (c1)) and in the presence ((a2), (b2), and (c2)) of cells.
- In the upper row free or conjugated neurotrophic factors (GDNF, βNGF, and FGF-2) were added to culture medium, each type separately (10 ng/mL, final concentration), and placed at 37°C (in the absence of cells).